-

-
A monoclonal antibody targeting the C-terminal of α-synuclein fibrils mitigatesFull Article
pathology in a Parkinson's disease model
Zeng S.Y.¶, Zhang S.N.¶, Gui X.¶, Li D.N.¶, Lv S.R., Dong H., Xu Q.H., Wu J., Ren H.Y., Zhao Q.Y., Long H.F., Yu Y.F., Hou S.Q., Le W.D., Fan M.Q., Liu D.T., Li D.*, Liu C.*
Cell Rep. 2025. (¶co-first author, *corresponding author) 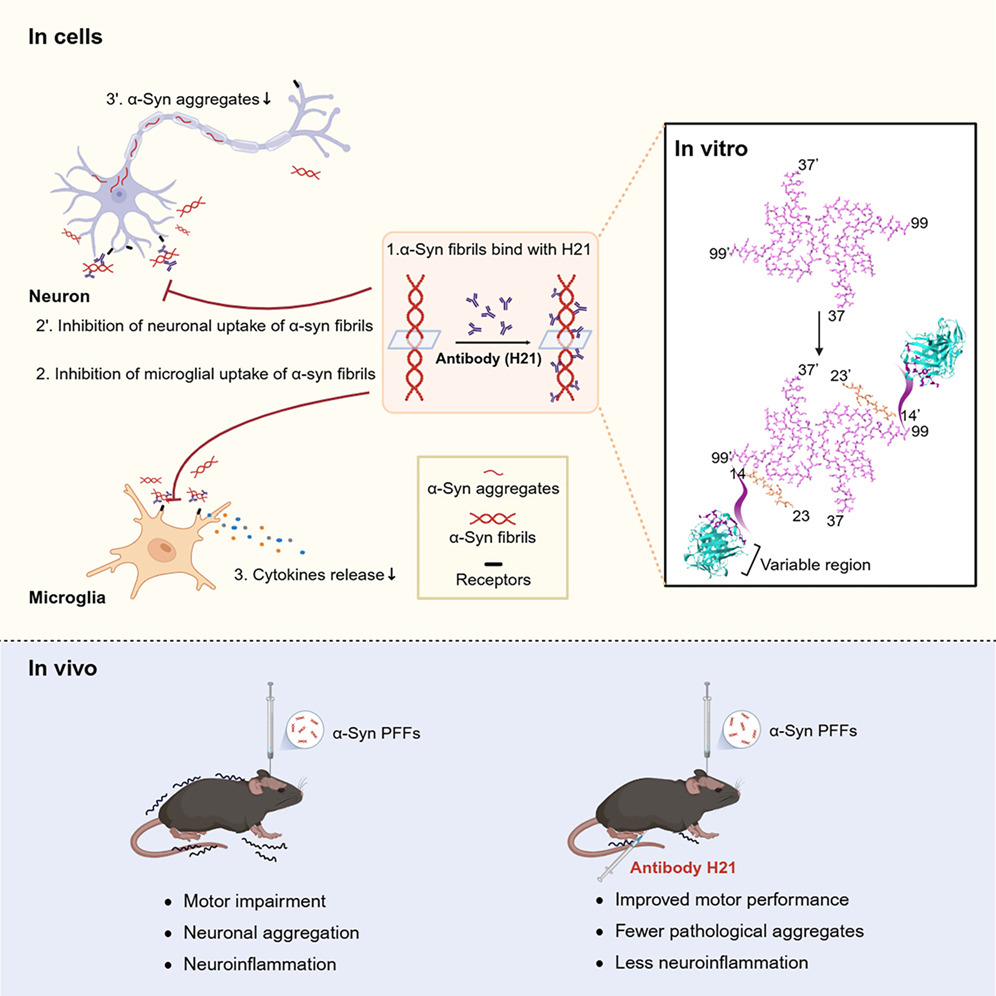
-

-
Dopamine-Induced Tau Modification Prevents Pathological Phosphorylation and Generates a Distinct Fibril PolymorphFull Article
Liu Z.T.¶, Li X.¶, Wang Q.W.¶, Liu K.E.¶, Zeng W., Li D.N., Zhao K., Ma Y.Y., Long H.F., Zhang S.N., Li D., Sun B., Le W.D., Wang C., He Z.H., Kang W.Y.*, Xiao W.D.*, Liu C.*
J Am Chem Soc. 2026. (¶co-first author, *corresponding author) 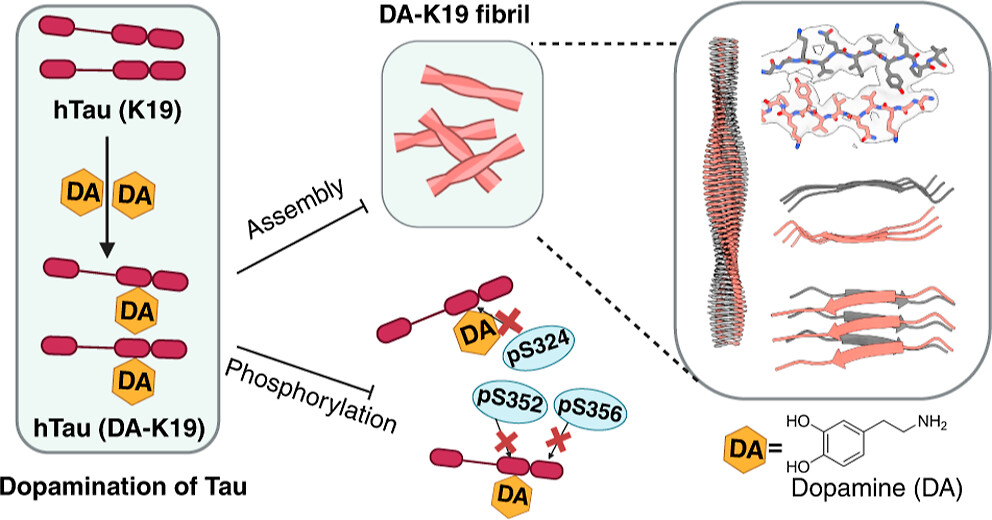
-

-
Biopsy-resolved cryo-EM structures of amyloid fibrils provide molecular insightsFull Article
into AL amyloidosis
Yao Y.X., Zhao Q.Y., Yao S., Xu Y.M., Liu K.E., Cao T.Y., Sun B., Zhou J.M.,
Liu C.*, Li D.*
Proc Natl Acad Sci U S A. 2026. (¶co-first author, *corresponding author) 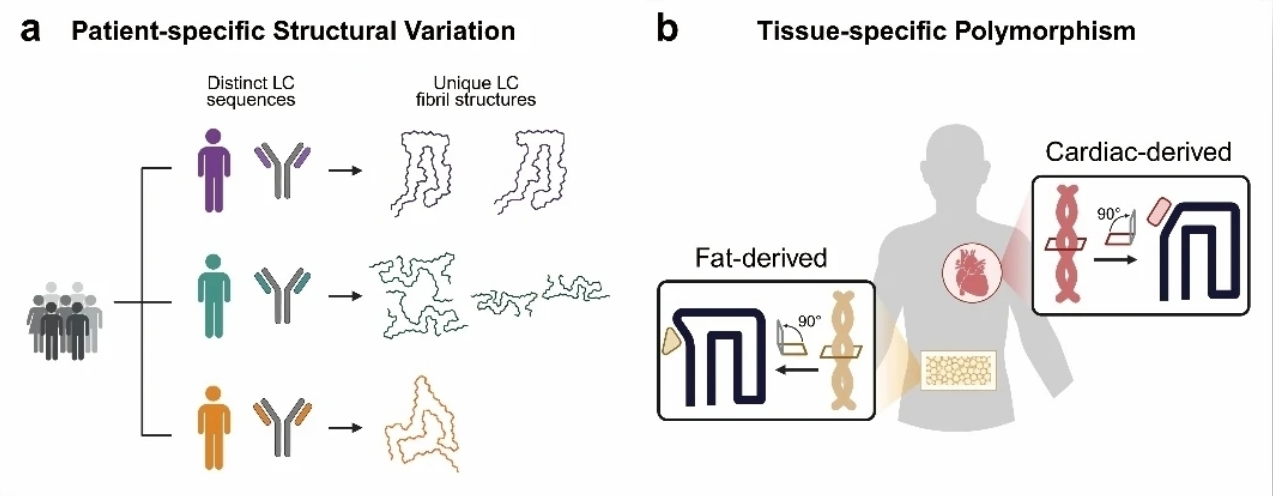
-

-
Conformational Adaptability and Thermostability in α/β-Peptide Fibrils InducedFull Article
by β-Amino Acid Substitution
Li Y.S.*, Li D.N.*, Yao Y.X., Liu.K.E., Zhao Q.Y., Zhang Y.L., Xu Y.Y., Li D., Sun B., Liu C.*, Dai B.*
Nano Lett. 2026. (¶co-first author, *corresponding author) 
-

-
Coexistence of G-Quadruplex and i-Motif Within a DNA Duplex is Tolerated byFull Article
a PCBP2-Assisted Replisome
Bao Y.L., Wu S.J., Gao L.J., Song X.X., Ren Z.Y., Zhao J.Z., Wang M.Y., Chen L.S.,
Cheng B.K., Hou X.M., Liu C.*, Sun Y.D*., Sun B.*
Angew Chem Int Ed Engl. 2026. (¶co-first author, *corresponding author) 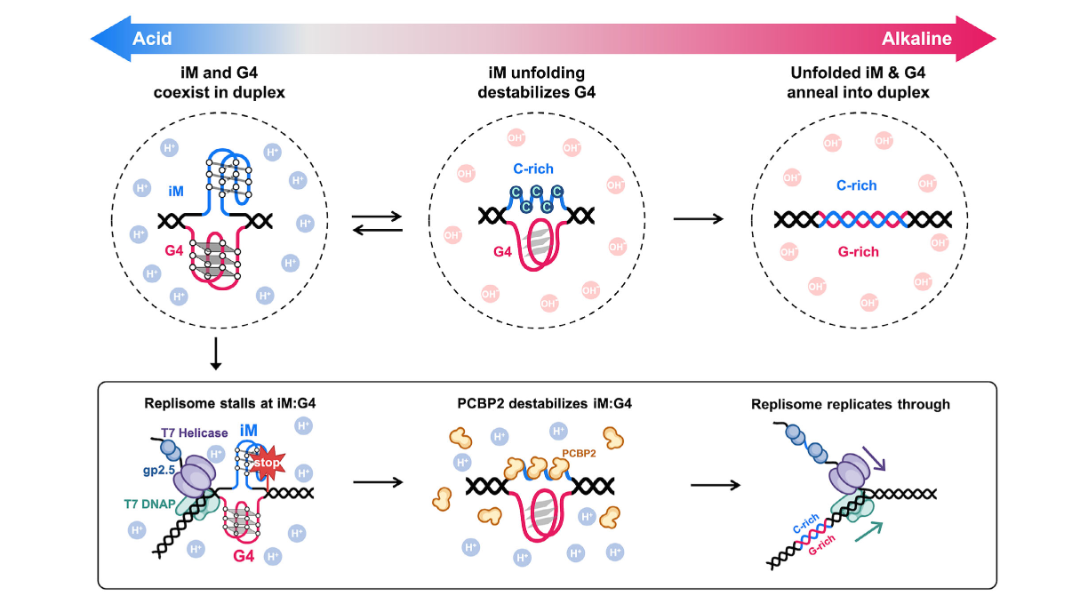
-

-
In-Situ Spatial Visual Proteomics Enabled by Single-Cell-Type ProximityFull Article
Biotinylation and Signaling Amplification
Zheng Z.D., Tang Z.Y., Li C., Xiao H.N., Li Y., He A., Mao Y.H., Zheng J.N., Ke M.,
Gao R., Li D., Liu C.*, Dong Z.*, Tian R.J.*
Angew Chem Int Ed Engl. 2026. (¶co-first author, *corresponding author) 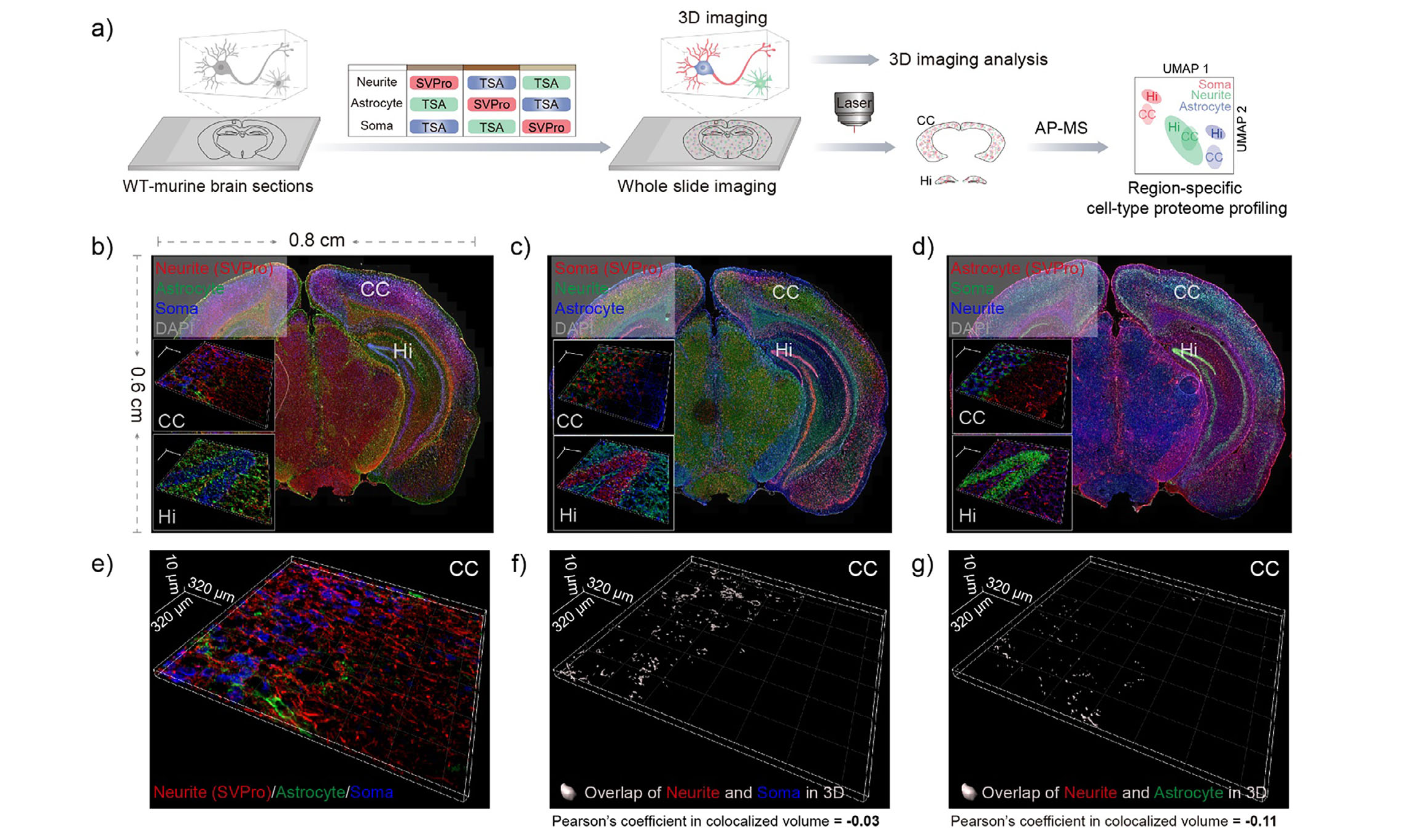
-

-
Atomic structure and in situ visualization of native PMEL lamellae inFull Article
melanosomes
Ma B.Y.¶, Yao Y.X.¶, Dong H.¶, Yang L.L.¶, Li D.N., Zhao Q.Y., Sun B., Chen Y.*,
Liu C.*, Li D.*
Nat Commun. 2025. (¶co-first author, *corresponding author) 
-

-
In situ amplification of α-synuclein amyloid fibril reveals a distinct polymorph related to Parkinson's disease and dementia with Lewy bodyFull Article
Cao T.Y.¶, Zhao Q.Y.¶, Yao Y.X., Liu K.E., Tao Y.Q., Lv S.R., Gao F., Shen Y., Ma C.,
Qiu W.Y., Liu C., Le W.D.*, Li D.*
Cell Rep. 2025. (¶co-first author, *corresponding author) 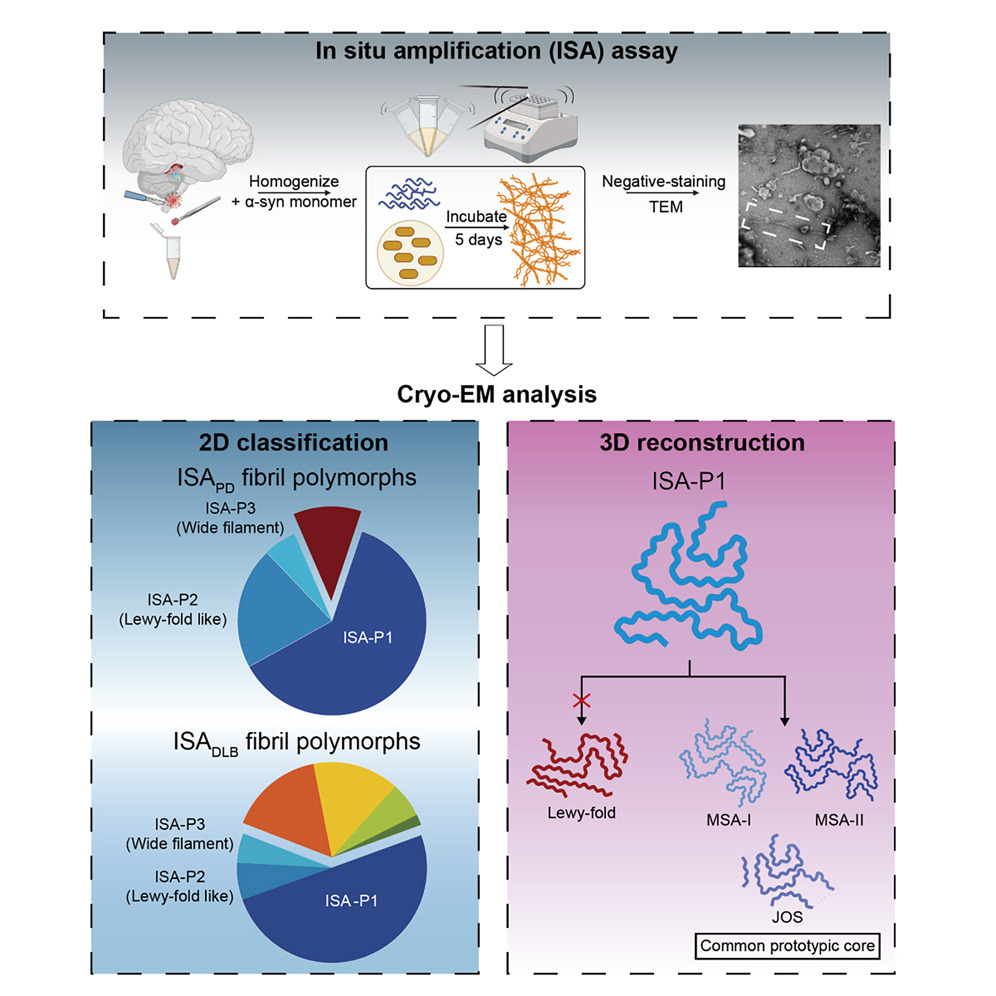
-

-
Full Article
Rising Stars: Molecular Mechanisms and Chemical Interventions of α-Synuclein
Zhang S.N.¶, Liu K.E.¶, Li D.*, Liu C.*
Amyloid Aggregation in Parkinson's Disease.
J Mol Biol. 2025. (¶co-first author, *corresponding author) 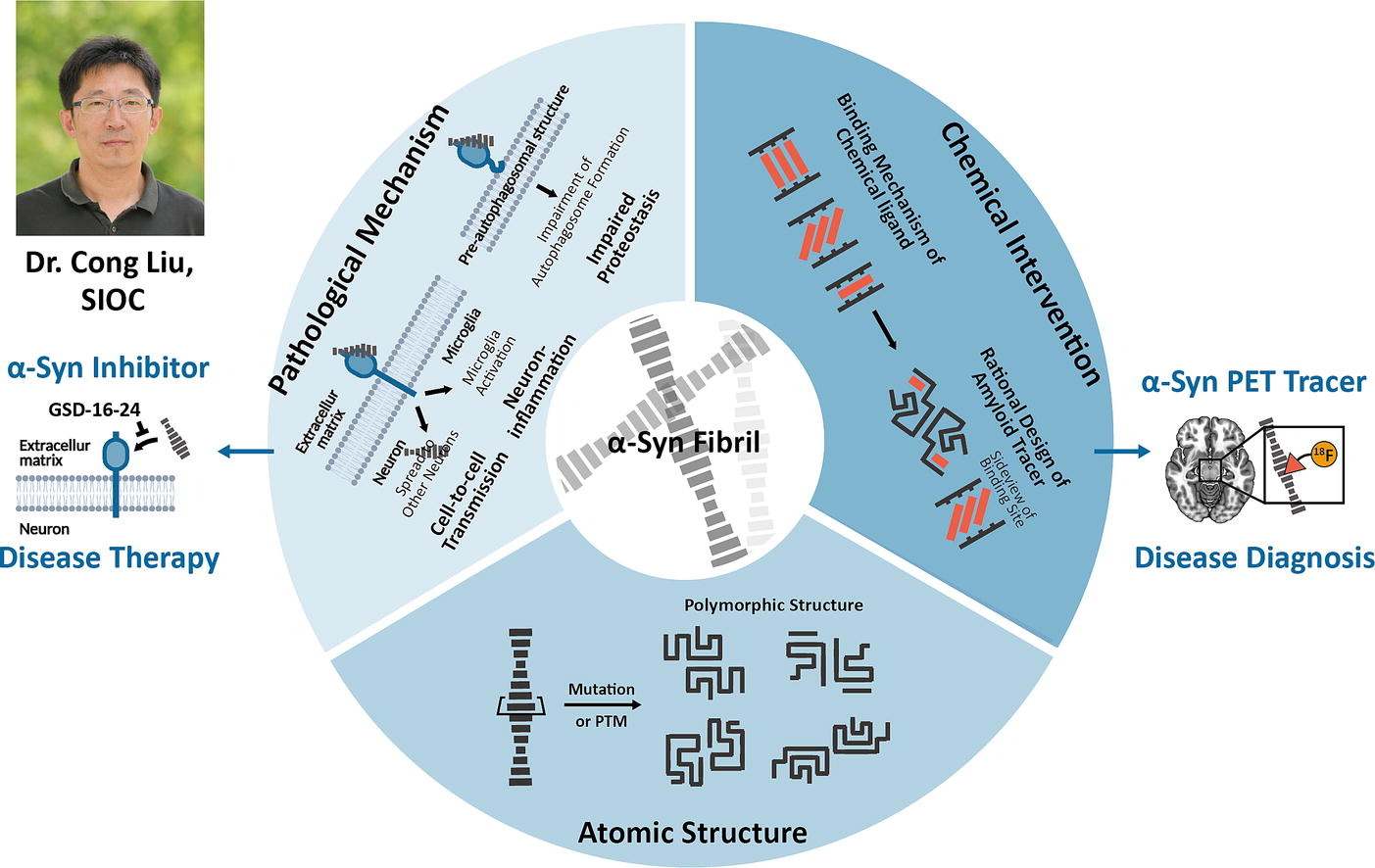
-

-
Amyloid Fibrillation of a Ninjurin-1-Derived α-Helical Peptide: Structural InsightsFull Article
into Conformational Transition
Wang M.J.¶, Xia W.C.¶, Zhao D., Zhai Z.Y., Chen R.J., Bai X., Zhang Z., Fan H.,
Zhang J.P., Liu C.*, Jiao F.*
ACS Nano. 2025. (¶co-first author, *corresponding author) 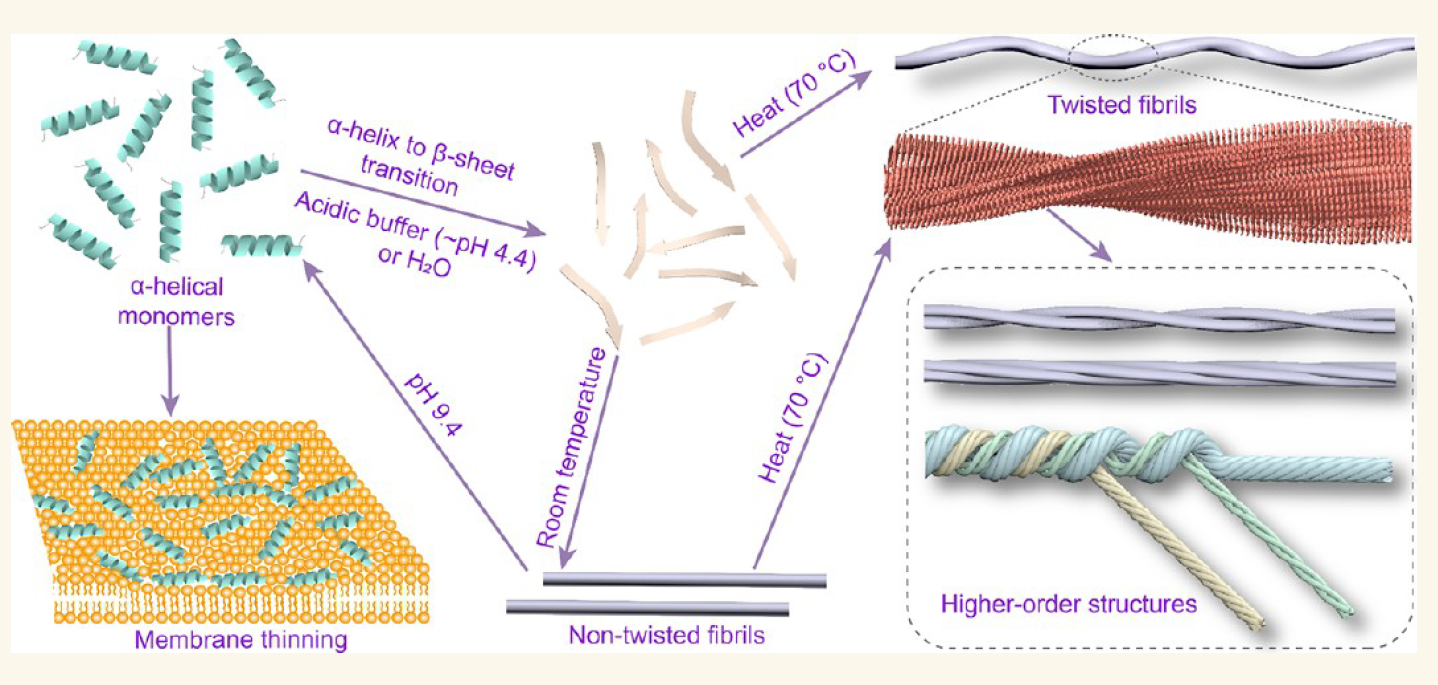
-

-
Intercellular propagation of RIPK1/RIPK3 amyloid fibrilsFull Article
Ma Y.Y.¶, Zhang Q.Y.¶, Li D.K., Zhao K., Li Z.F., Liu Y., Wang C., Sun B., Li D.,
Yuan J.Y.*, Liu C.*
Proc Natl Acad Sci U S A. 2025. (¶co-first author, *corresponding author) 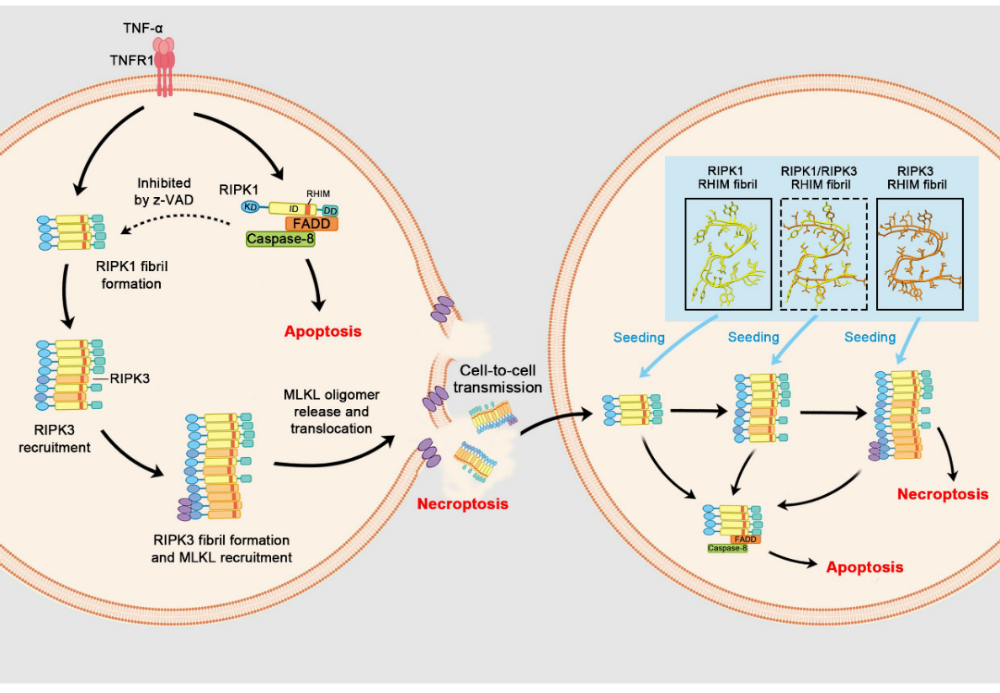
-
-
De novo design of protein binders to stabilize monomeric TDP-43 and inhibitFull Article
its pathological aggregation
Sun G.Y.¶, Li X.¶, Hu J.J., Yang T.B., Liu C.*, Wang Z.Z.*, Li D.*, Xu W.C.*
Proc Natl Acad Sci U S A. 2025. (¶co-first author, *corresponding author) 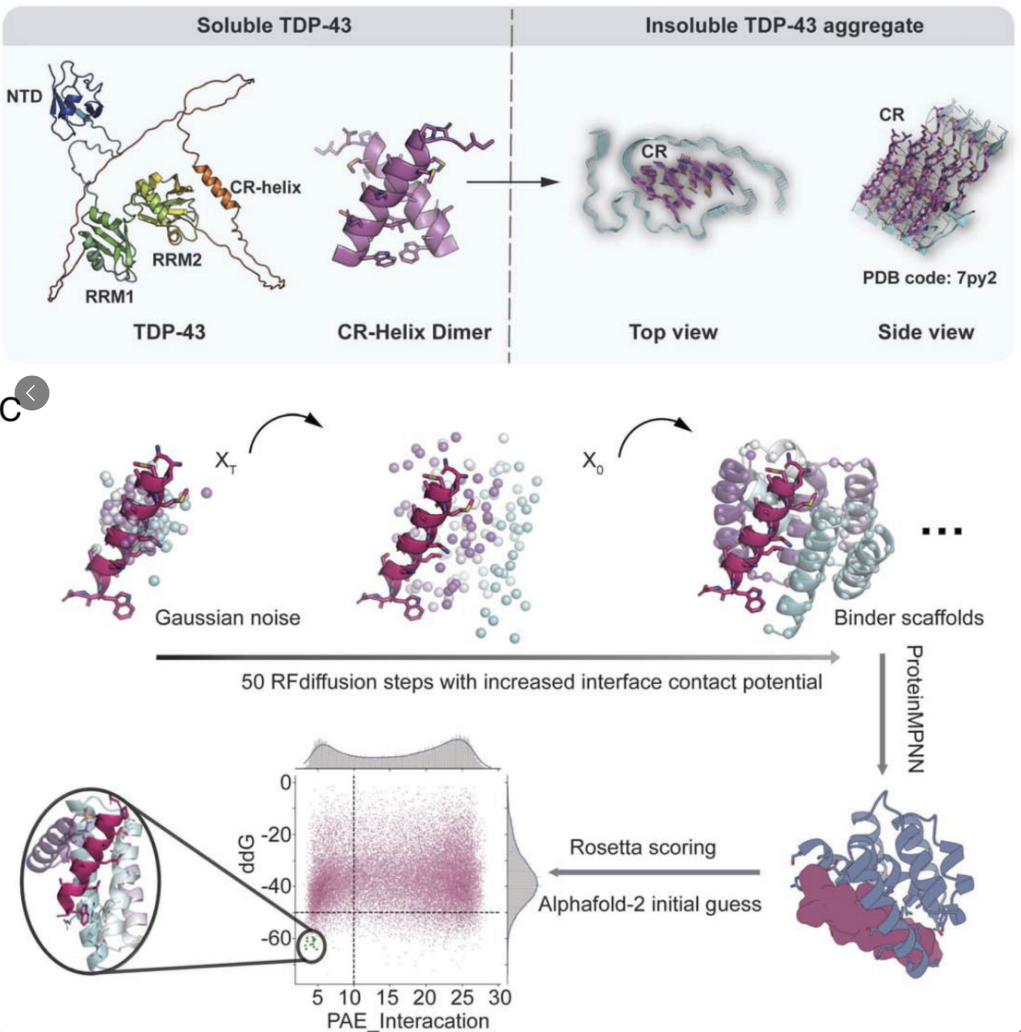
-

-
Distinct amyloid fibril structures formed by ALS-causing SOD1 mutants G93A and D101NFull Article
Zhang M.Y.¶, Ma Y.Y.¶, Wang L.Q.¶, Xia W.C., Li X.N., Zhao K., Chen J., Zou L.Y.,
Wang Z.Z., Liu C.*, Liang Y.*
EMBO Rep. 2025. (¶co-first author, *corresponding author) 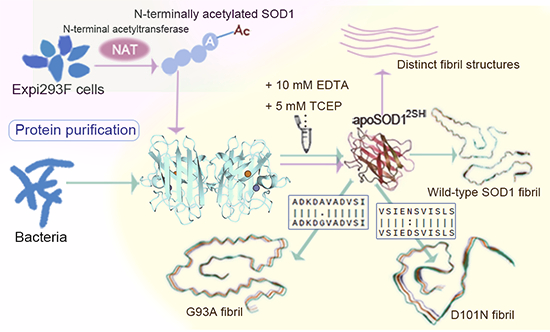
-

-
HSF-1 Regulates Autophagy to Govern Motor Function and Facilitate ToxicFull Article
Protein Clearance in a C. elegans Model of Amyotrophic Lateral Sclerosis
Xu H.*¶, Shao Y.P., Zhang J., Ni Y., Xu G.W., Liu C., Liang Y., Le W.D.*
Neurosci Bull. 2025. (¶co-first author, *corresponding author) 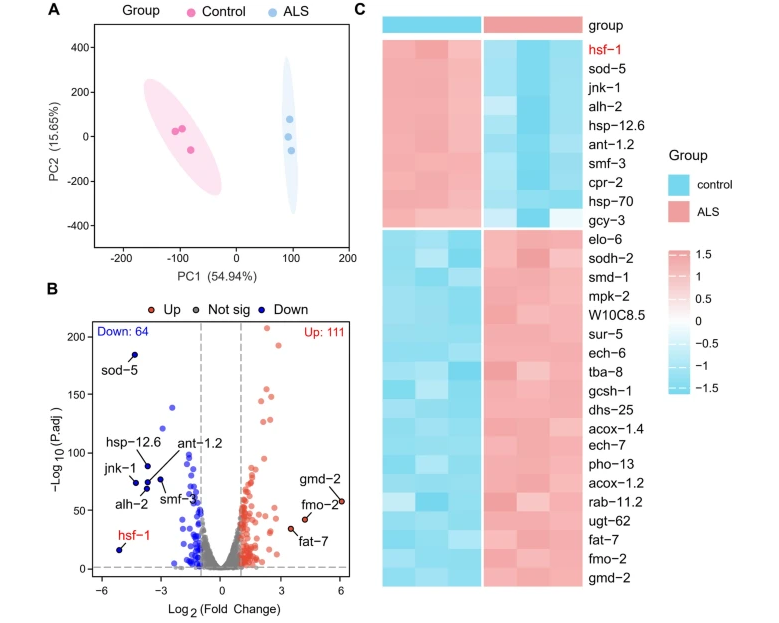
-

-
Design of Ig-like binders targeting α-synuclein fibril for mitigating itsFull Article
pathological activities
Zeng S.Y.¶, Xiong X.Y., Long H.F., Xu Q.H., Yu Y.F., Sun B., Liu C., Wang Z.Z., Xu W.Q., Zhang S.N., Li D.*
Nat Commun. 2025. (¶co-first author, *corresponding author) 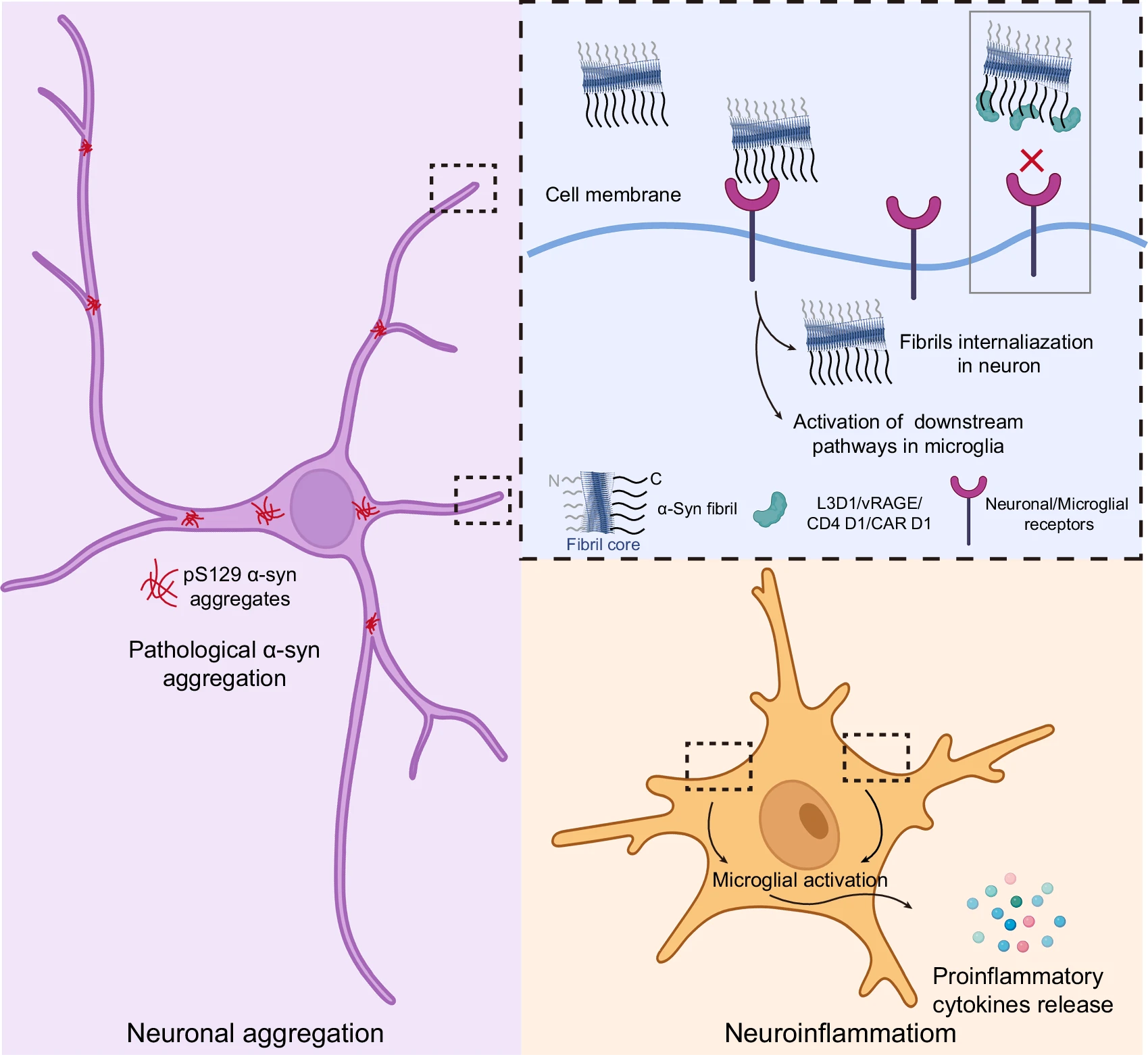
-

-
An O-glycopeptide participates in the formation of distinct Aβ42 fibril structures and attenuates Aβ42 neurotoxicityFull Article
Wei Q.J.¶, Liu D.L.¶, Xia W.C.¶, Wang F.Z., Lu H., Zhang J., Wang X.Y., Xu Z.X.,
He C.D., Li W.Z., Shi X.M., Wang C., Liu Y.*, Liu C.*, Dong S.W.*
Nat Commun. 2025. (¶co-first author, *corresponding author) 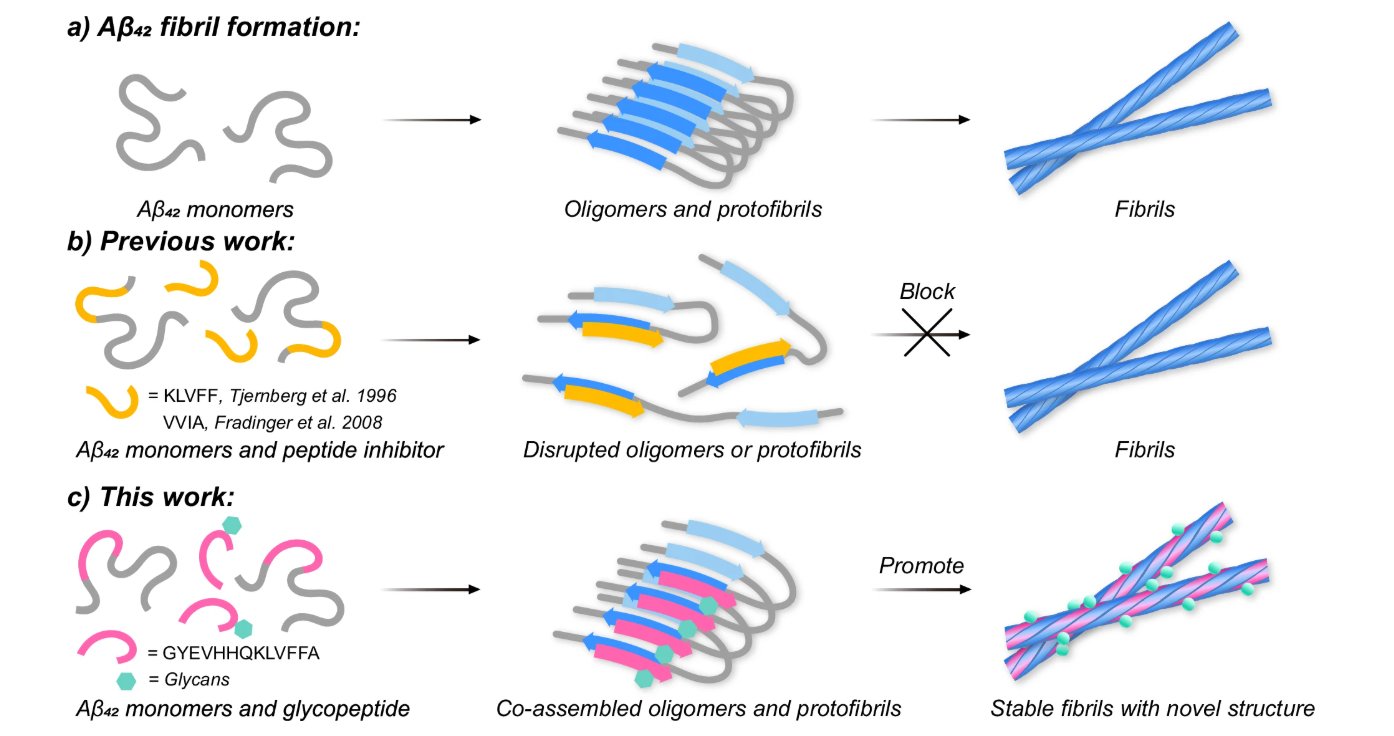
-

-
Voluntary wheel running exercise improves sleep disorder, circadian rhythmFull Article
disturbance, and neuropathology in an animal model of Alzheimer's disease
Hu Y.Y.¶, Niu L., Chen Y.X., Yang H.J., Qiu X.H., Jiang F., Liu C., Cai H.B., Le W.D.*
Alzheimers Dement. 2025. (¶co-first author, *corresponding author) 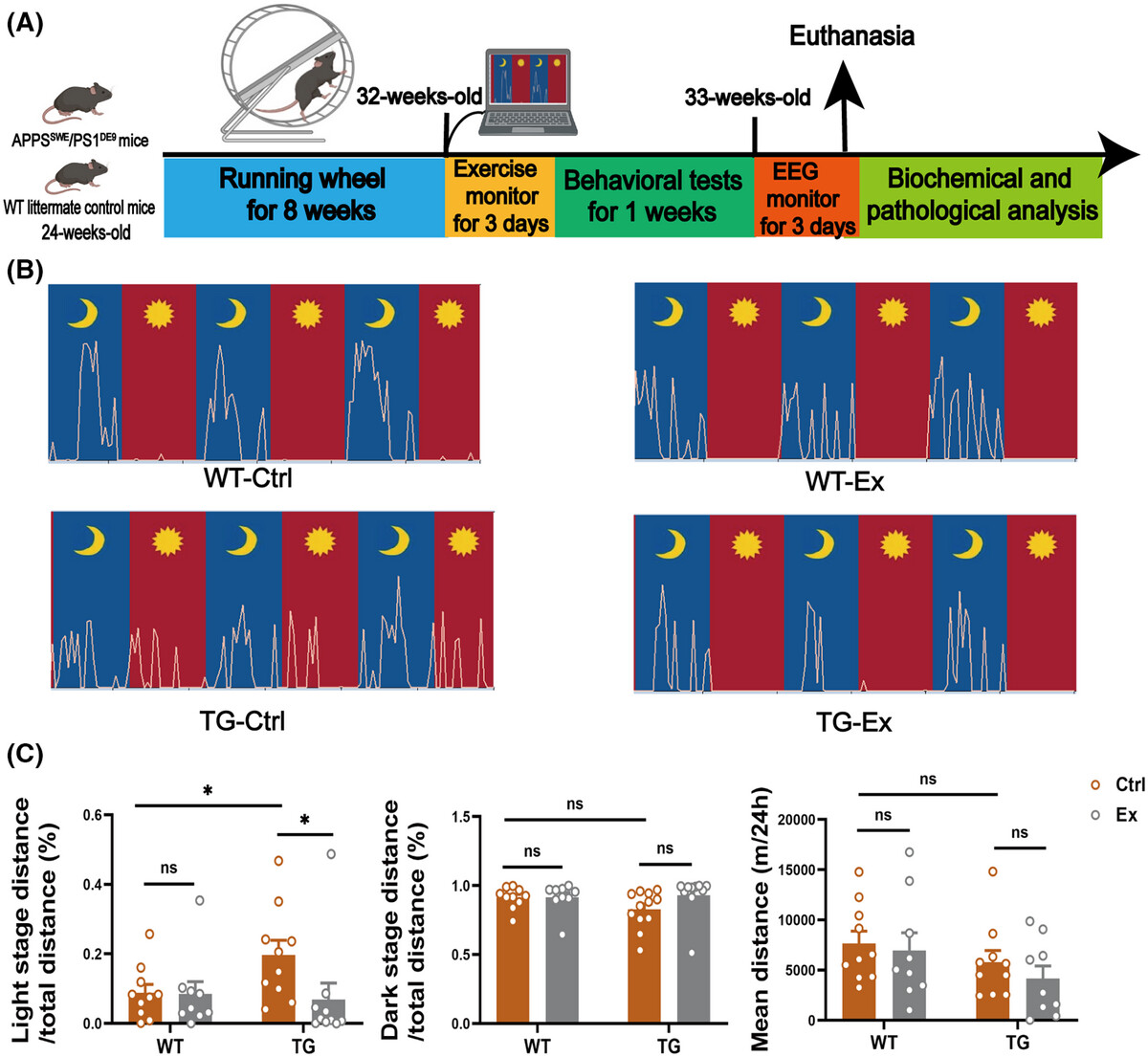
-
-
YAP maintains the dynamics of TDP-43 condensates and antagonizes TDP-43Full Article
pathological aggregates
Zhang J.Q.¶, Hu J.J.¶, Liu R.G.¶, Zhou T., Luo X.W., Liang P.G., Xie Z.C., Zhao Q.Y.,
Chen Y., Du D., Liu C., Zheng Y.M.*, Li D.*, Wang Bo.*
Nat Cell Biol., 2025. (¶co-first author, *corresponding author) 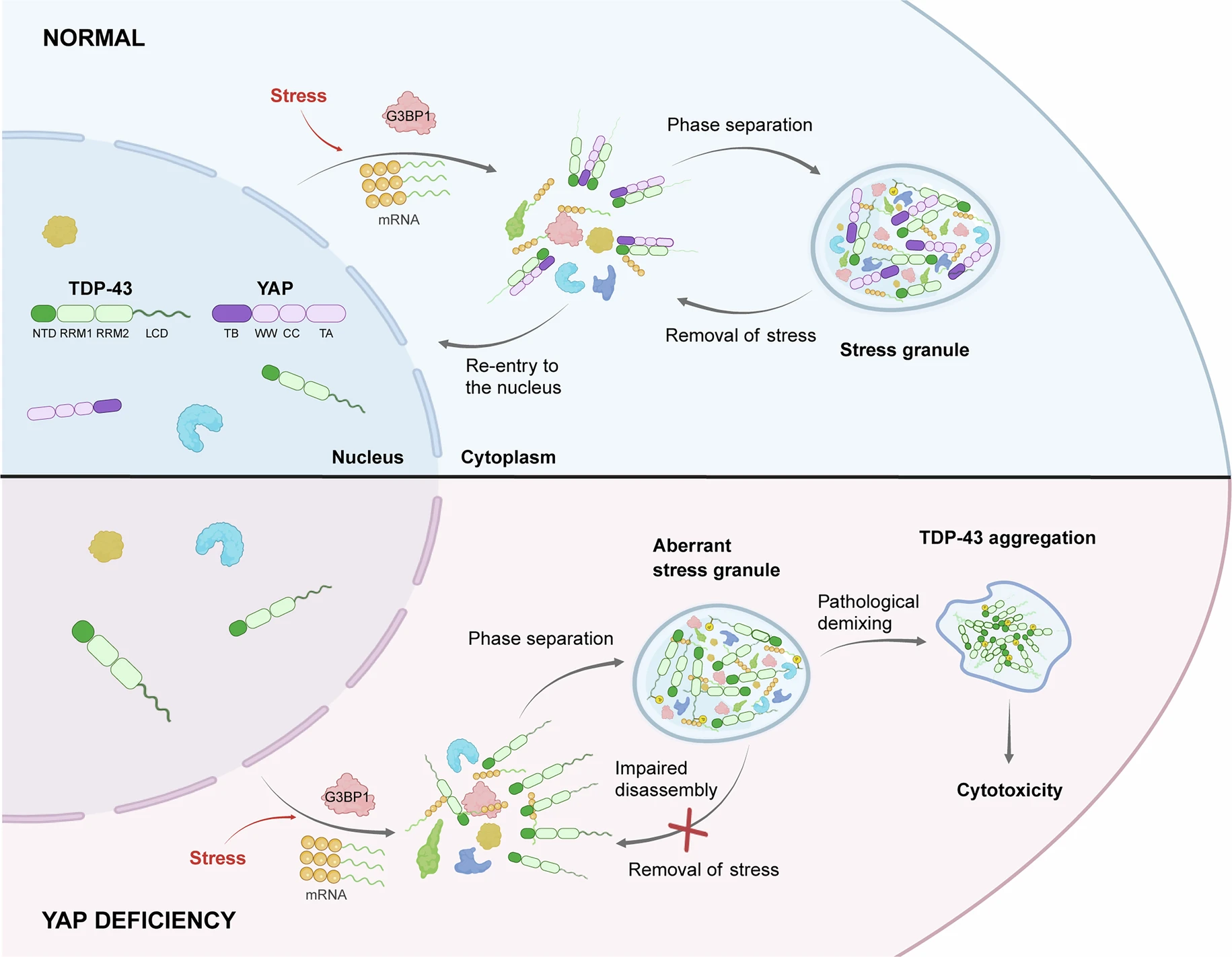
-

-
Unraveling Alzheimer's complexity with a distinct Aβ42 fibril type and specificFull Article
AV-45 binding
Zhao Q.Y.¶, Tao Y.Q.¶, Yao Y.X., Liu K.E., LV S.R., Cui B.Y., Xiao W.D., Cao T.Y., Li W.D., Gao F., Shen Y., Wang C., Ma C., Qiu W.Y., Liu C.*, Li D.*
Nat Chem Biol., 2025. (¶co-first author, *corresponding author) 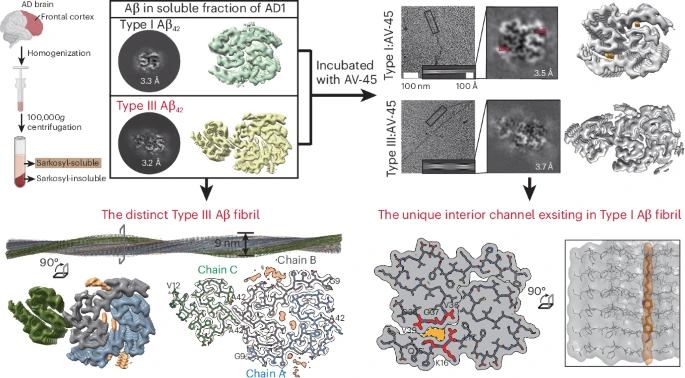
-

-
Design and Structural Elucidation of Glycopeptide Fibrils: Emulating Glycosaminoglycan Functions for Biomedical ApplicationsFull Article
Xia W.C.¶, Xu Z.X.¶, Dong H., Zhang S.N., He C.D., Li D., Sun B., Dai B., Dong S.W.*, Liu C.*
J Am Chem Soc., 2025. (¶co-first author, *corresponding author) 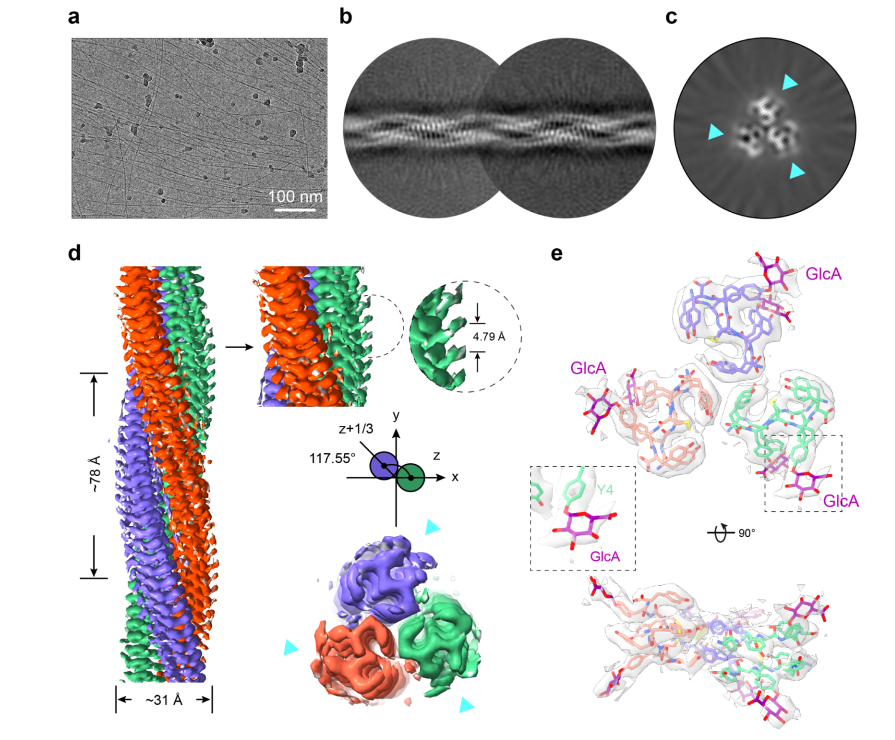
-
-
MEK1/2 inhibitors suppress pathological α-synuclein and neurotoxicity in cellFull Article
models and a humanized mouse model of Parkinson’s disease
Wang H.L.¶, Wang Q.¶, Xu H.X.¶, Wu Y.Z., Siulam C., Xu Q.H., Pan C.F., Cao J.Y.,
Cao Z.Y., Yang R.N., Ding Y., Fei Y.Y., Chen Y.F., Wang J.*, Liu C.*, Lu B.X.*
Sci Transl Med., 2025. (¶co-first author, *corresponding author) 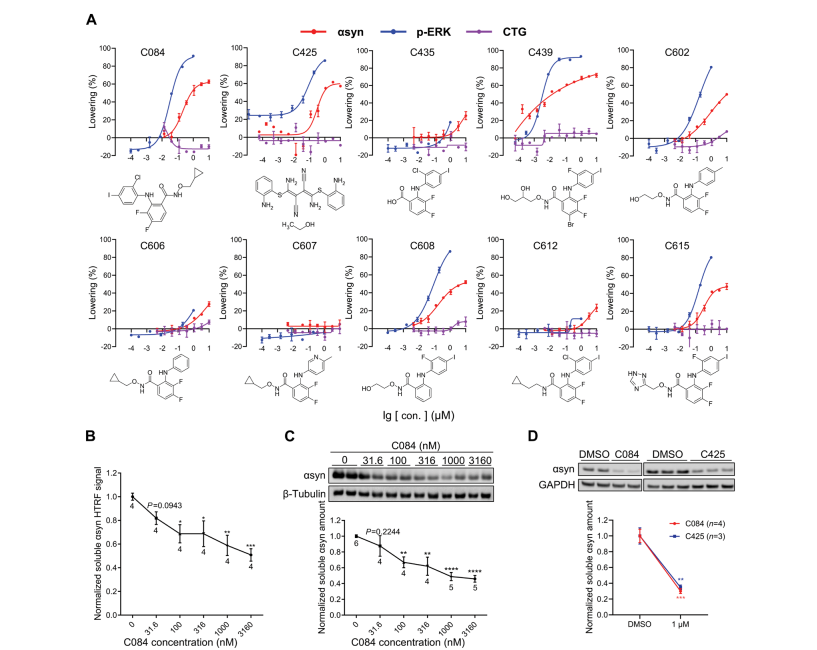
-

-
Biological Effects of Dietary Restriction on Alzheimer's Disease: Experimental and Clinical InvestigationsFull Article
Liu Z.J.¶, Zhang J., Jiang F., Liu C., Shao Y.P., Le W.D.*
CNS Neurosci Ther., 2025. (¶co-first author, *corresponding author) 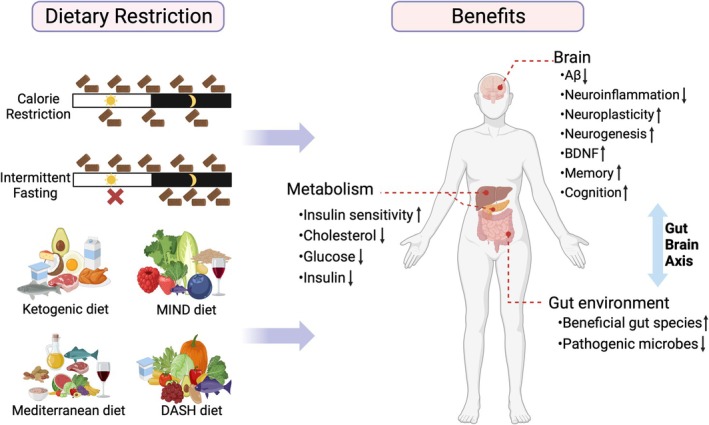
-

-
Fibril fuzzy coat is important for α-synuclein pathological transmission activityFull Article
Han Y.L., Li J., Xia W.C., li Q.T., Sun Z.H., Zeng W., Hu Y.X., Kelvin C.Luk., Liu C.*,
Xia S.Q.*, He Z.H.*
Neuron., 2025. (¶co-first author, *corresponding author) 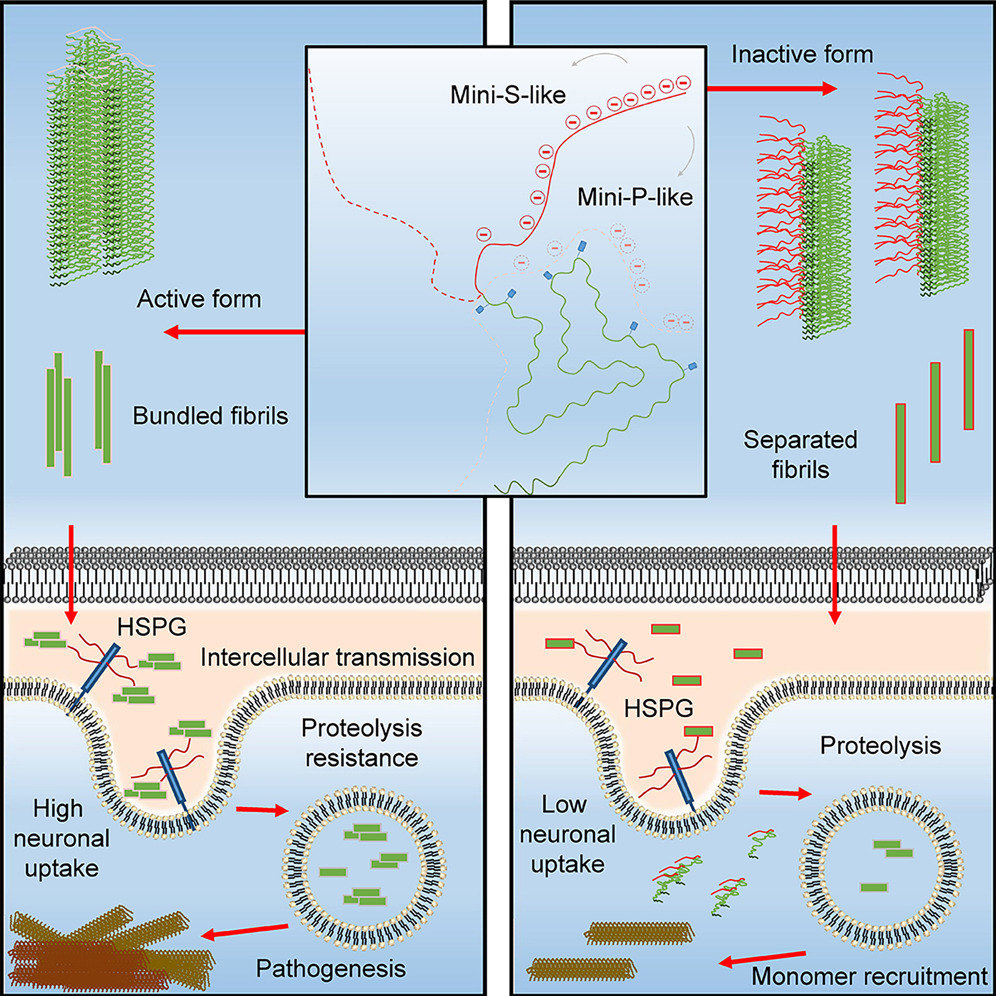
-

-
β-Lactoglobulin Forms a Conserved Fibril Core That Assembles into Diverse Fibril PolymorphsFull Article
Xu Y.Y.¶, Li D.N.¶, Zhang Y.L., Zhao Q.Y., Sun B., Liu C., Li D., Dai B.*
Nano Lett., 2025. (¶co-first author, *corresponding author) 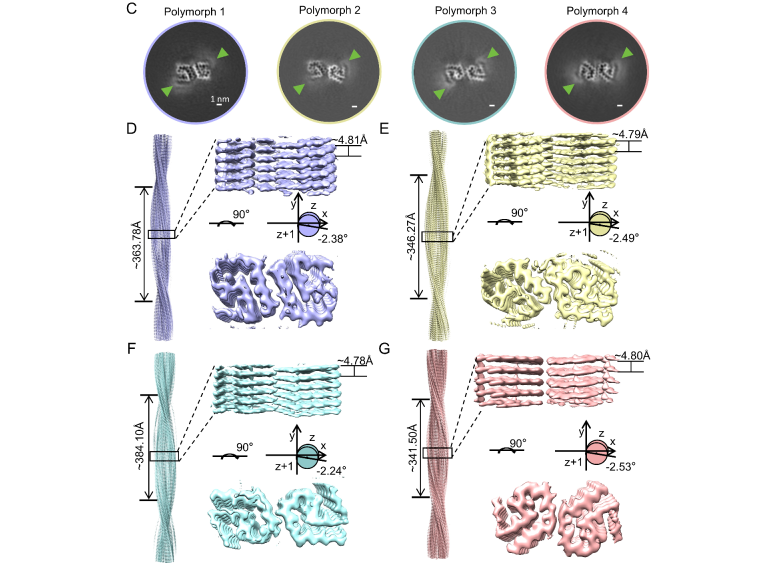
-

-
Quantitative chemoproteomics reveals dopamine's protective modification of TauFull Article
Wang Q.W.¶, Liu Z.T.¶, Wang Y.J.¶, Liu Y., Chen Y., Zhang S.N., Zeng W., Li D.,
Yang F., He Z.H., Xiao W.D.*, Liu C.*, Wang C.*
Nat Chem Biol., 2025. (¶co-first author, *corresponding author) 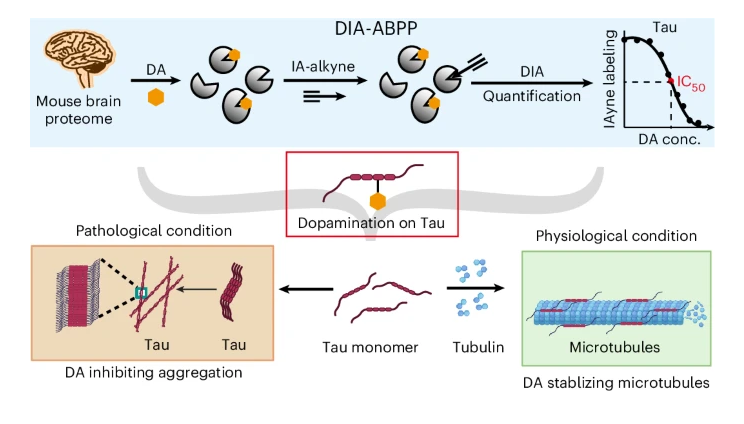
-

-
Neuronal FAM171A2 mediates α-synuclein fibril uptake and drives Parkinson'sFull Article
disease
Wu K.M.¶, Xu Q.H., Liu Y.Q., Feng Y.W., Han S.D., Zhang Y.R., Chen S.D.,
Guo Y., Wu B.S., Ma L.Z., Zhang Y., Chen Y.L., Yang L., Yao Y.F., Xiao Y.J.,
Wang T.T., Zhao J., Chen S.F., Cui M., Lu B.X., Le W.D., Shu Y.S., Ye K.Q.,
Li J.Y., Li W.S., Wang J., Liu C.*, Yuan P.*, Yu J.T.*
Science., 2025. (¶co-first author, *corresponding author) 
-

-
Full Article
Single-Molecule Insight Into α-Synuclein Fibril Structure and Mechanics
Modulated by Chemical Compounds
Li X.¶, Bi L.L.¶, Zhang S.Q., Xu Q.H., Xia W.C., Tao Y.Q., Wu S.J., Li Y.N., Le W.D.,
Kang W.Y., Li D., Sun B.*, Liu C.*
Adv Sci., 2025. (¶co-first author, *corresponding author) 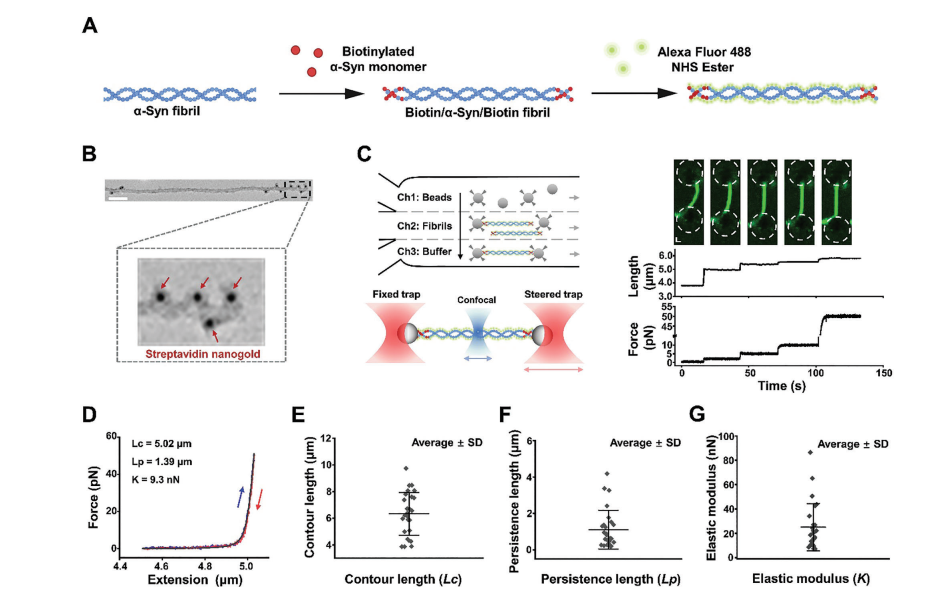
-

-
Tracing TMEM106B fibril deposition in aging and Parkinson's disease withFull Article
dementia brains
Zhao W.B.¶, Fan Y.¶, Zhao Q.Y., Fan Z., Zhao J., Yu W.B., Li W.S., Li D.,
Liu C.*, Wang J.*
Life Med., 2024. (¶co-first author, *corresponding author) 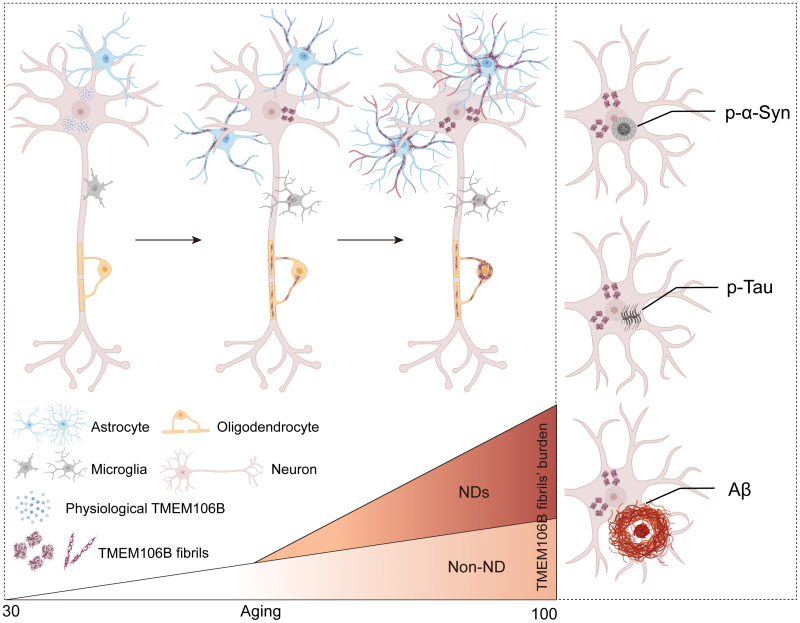
-

-
LncRNAs chaperoning dynamic protein condensates in cancer cellsFull Article
Hu J.J.¶*, Li D., Liu C.*
Mol Cell., 2024. (¶co-first author, *corresponding author) 
-
-
N-acetylation of α-synuclein enhances synaptic vesicle clustering mediated byFull Article
α-synuclein and lysophosphatidylcholine
Wang C.C.¶, Zhao C.Y.¶, Xiao H., Qiang J.L., Liu Z.Y., Gu J.G., Zhang S.N., Li D.,
Zhang Y.Y., Burré J., Diao J.J.*, Liu C.*
Elife., 2024. (¶co-first author, *corresponding author) 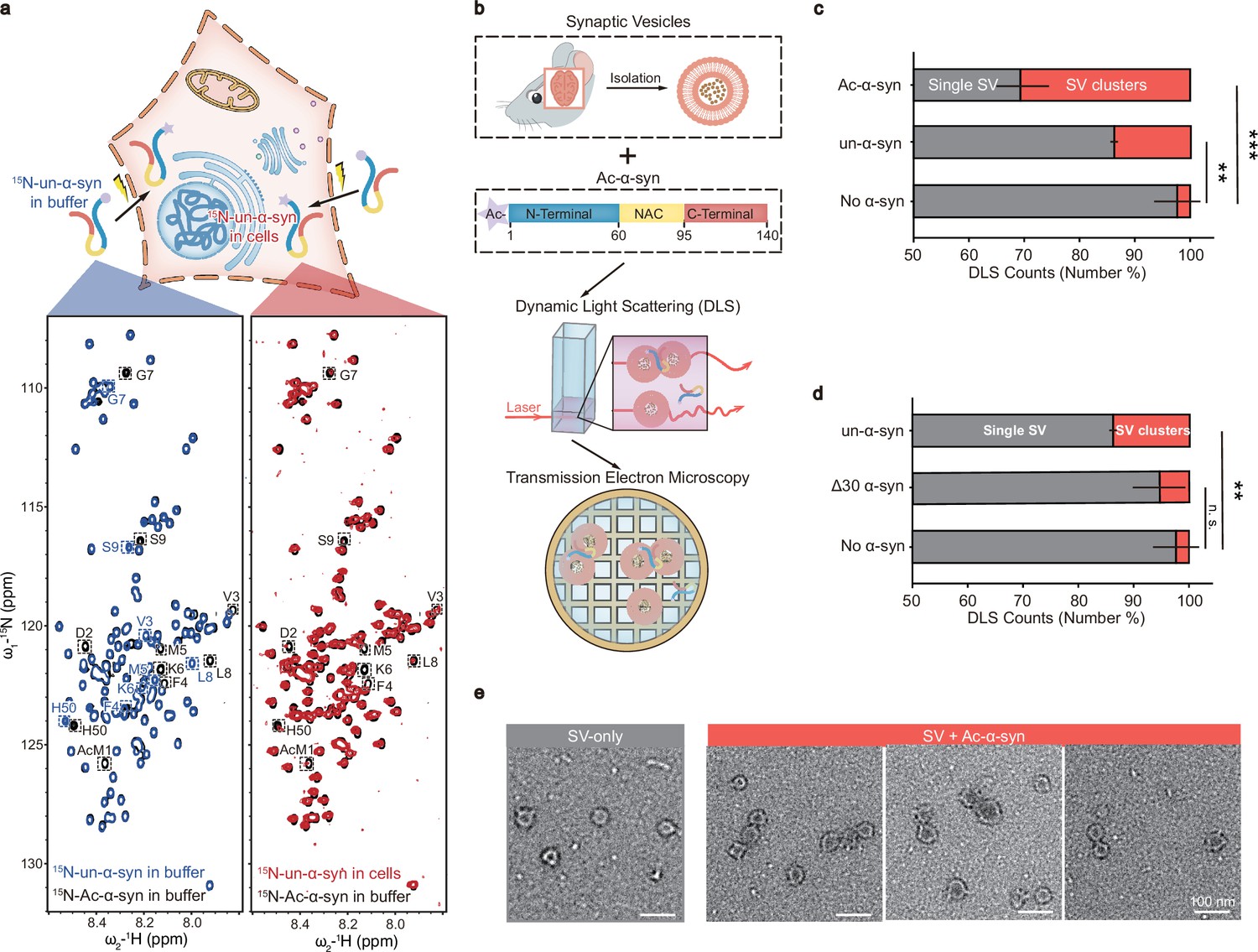
-

-
Integrated cerebellar radiomic-network model for predicting mild cognitiveFull Article
impairment in Alzheimer's disease
Chen Y.N., Qi Y.W., Hu Y.Y., Qiu X.H., Qiu T., Li S., Liu M.C., Jia Q.Q., Sun B., Liu C.,
Li T.B., Le W.D.
Alzheimers Dement., 2024. (¶co-first author, *corresponding autho 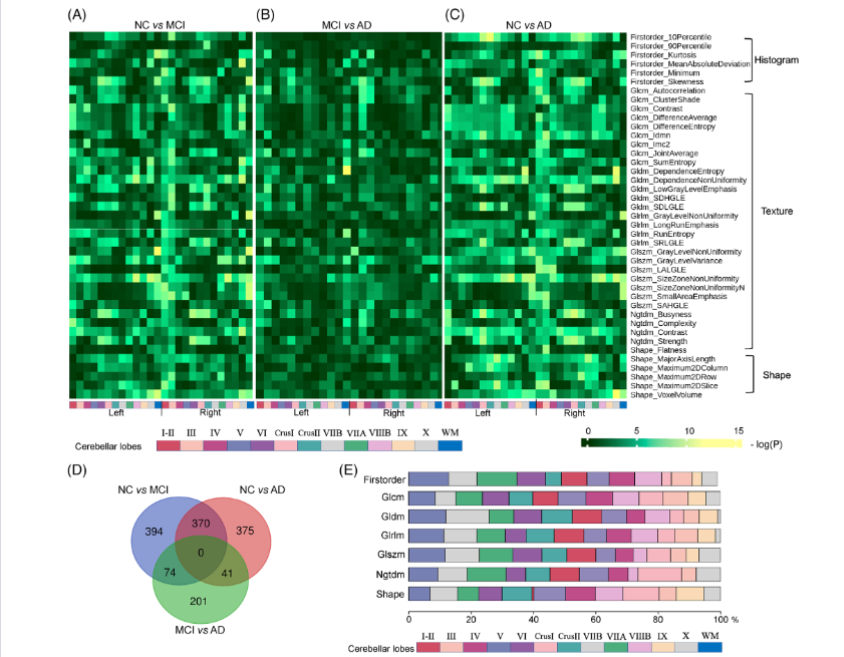
-

-
CRISPR-AsCas12f1 couples out-of-protospacer DNA unwinding with exonuclease activity in the sequential target cleavageFull Article
Song X.X.¶, Chen Z.T.¶, Sun W.J., Yang H., Guo L.J., Zhao Y.L., Li Y.N., Ren Z.Y., Shi J., Liu C., Ma P.X., Huang X.X., Ji Q.J., Sun B.*
Nucleic Acids Res., 2024. (¶co-first author, *corresponding author) 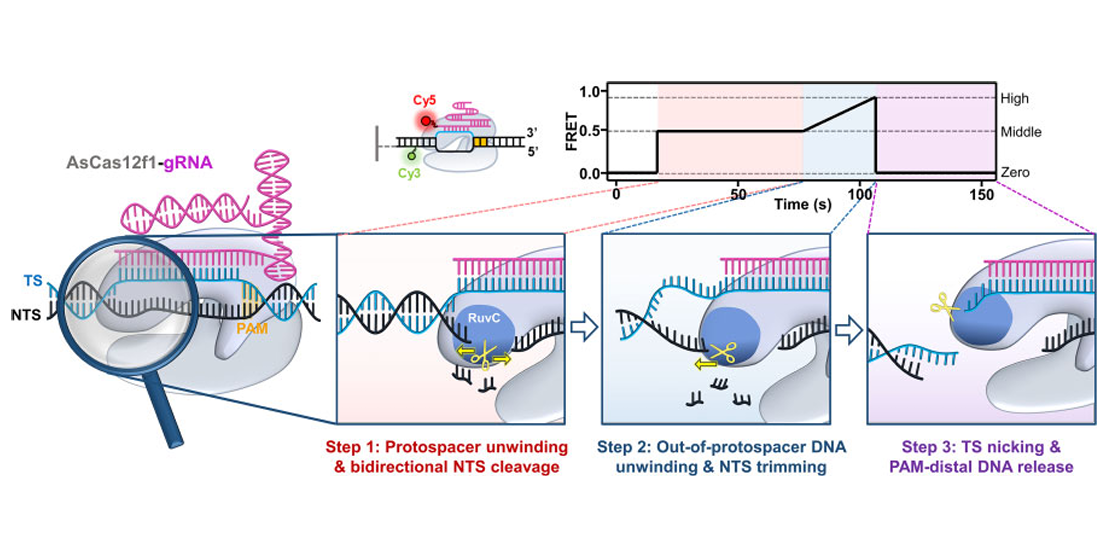
-

-
Amyloid fibril structures and ferroptosis activation induced by ALS-causing SOD1 mutationsFull Article
Wang L.Q.¶, Ma Y.Y.¶, Zhang M.Y.¶, Yuan H.Y., Li X.N., Xia W.C., Zhao K., Huang X., Chen J., Li D., Zou L.Y., Wang Z.Z., Le W.D., Liu C.*, Liang Y.*
Sci Adv., 2024. (¶co-first author, *corresponding author) 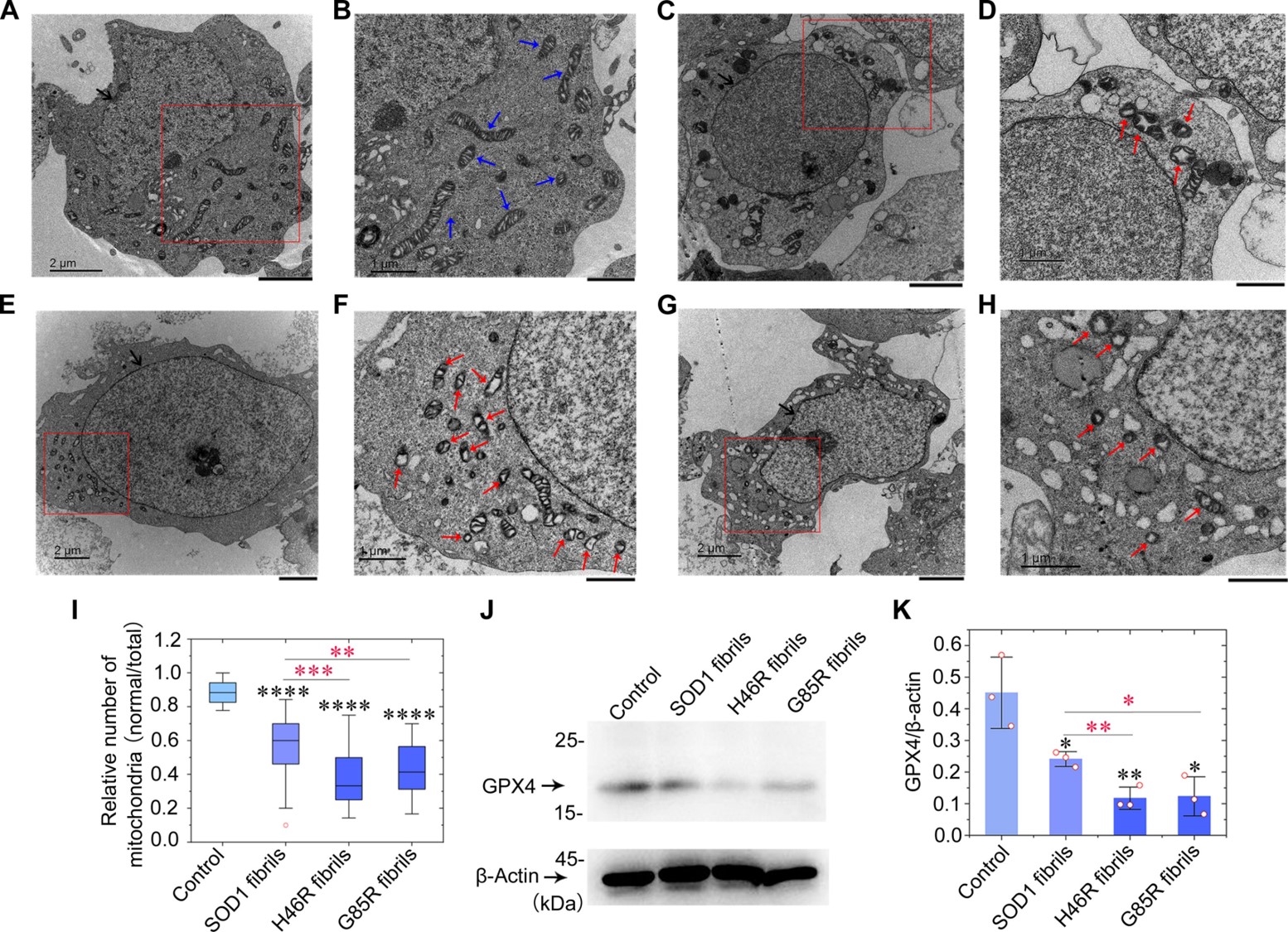
-

-
Time-course remodeling and pathology intervention of α-synuclein amyloid fibril by heparin and heparin-like oligosaccharidesFull Article
Tao Y.Q.¶, Xu P.¶, Zhang S.Q., Wei S.G., Yang G., Liu K.E., Li X., Sun Y.P., Zhao Q.Y.,
Li D., Yu B.*, Liu C.*
Nat Struct Mol Biol., 2024. (¶co-first author, *corresponding author) 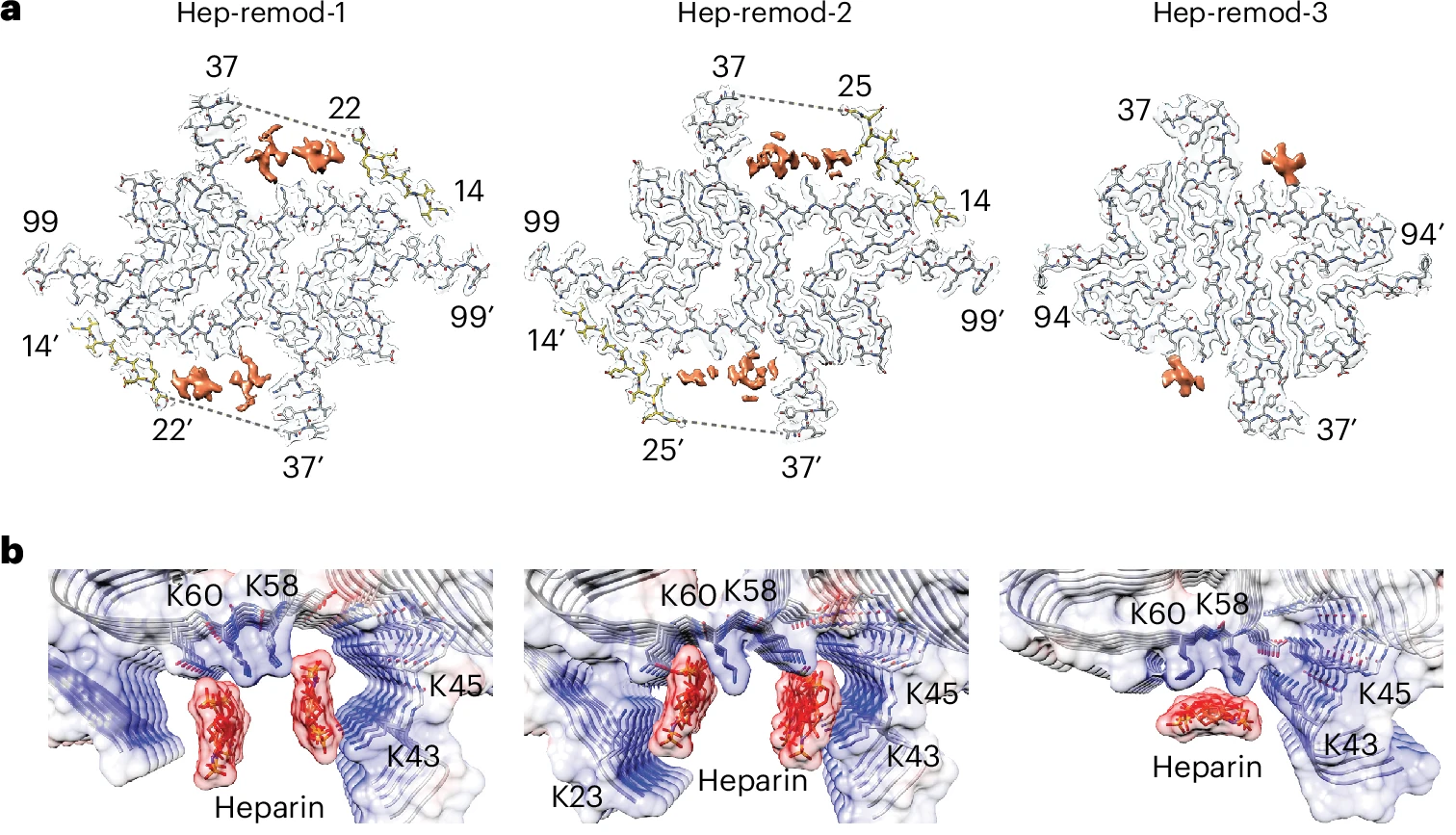
-

-
Homologous Peptide Foldamer Promotes FUS Aggregation and Triggers CancerFull Article
Cell Death
Wang M.D.¶, Yi L., Li Y.Y., Xu R.W., Hu J.J., Hou D.Y., Liu C.*, Wang H.*
J Am Chem Soc., 2024. (¶co-first author, *corresponding author) 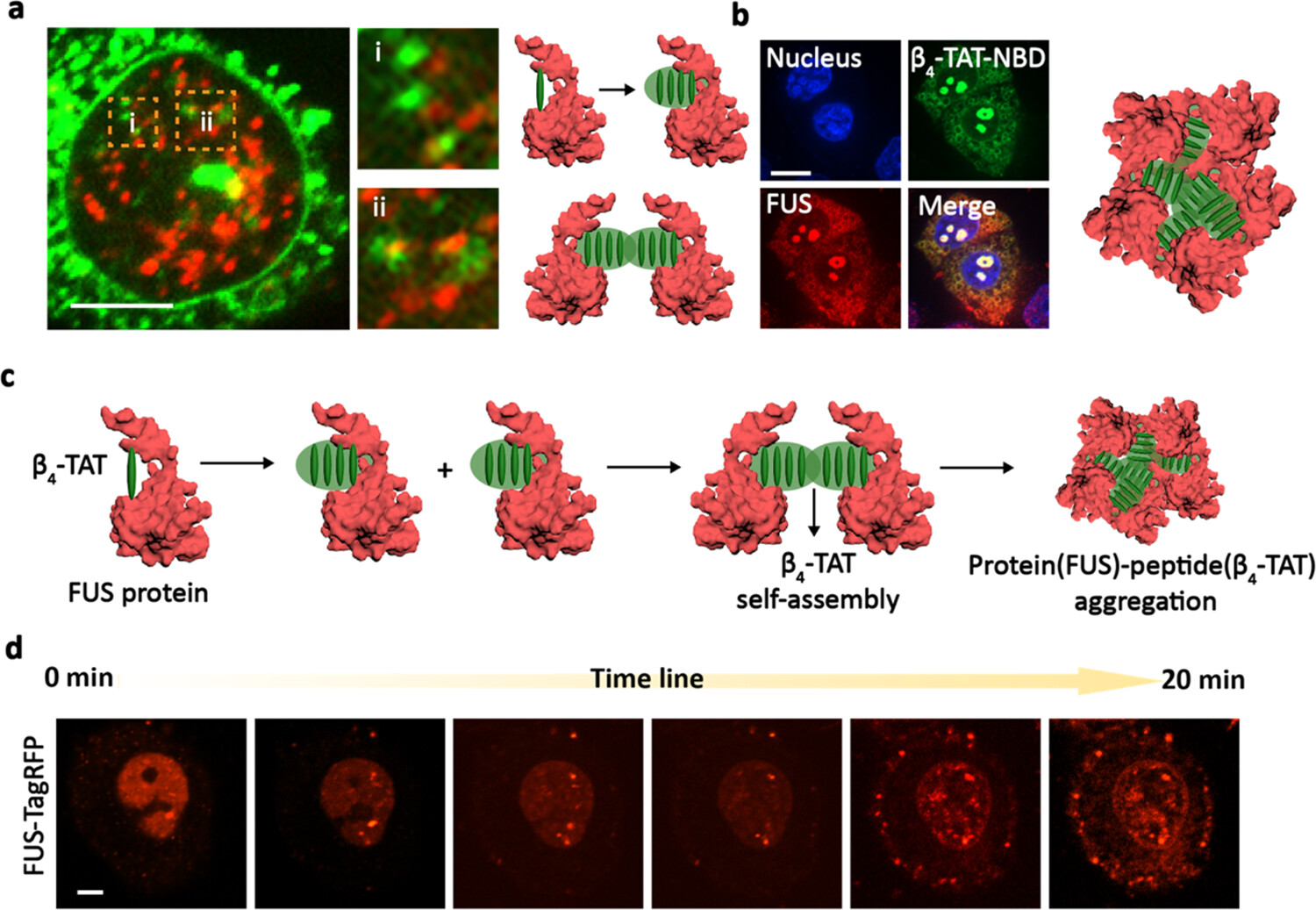
-

-
Self-limiting multimerization of α-synuclein on membrane and its implication inFull Article
Parkinson's diseases
Ma D.F.¶, Zhang S.Q.¶, Xu S.Y.¶, Huang Z., Tao Y.X., Chen F.Y., Zhang S.N., Li D.,
Chen T.S, Liu C.*, Li M.*, Lu Y.*
Sci Adv., 2024. (¶co-first author, *corresponding author) 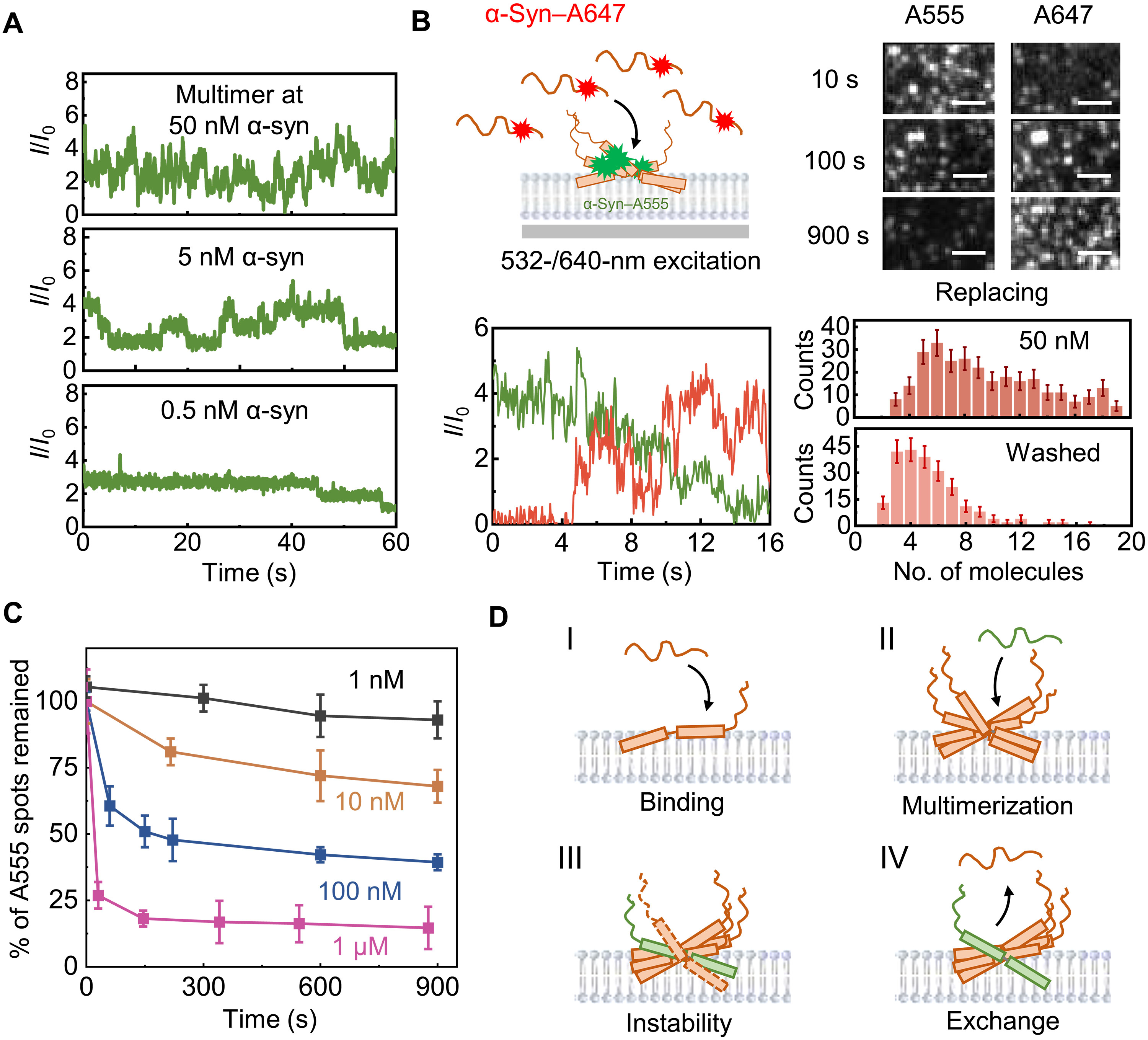
-

-
Different charged biopolymers induce α-synuclein to form fibrils with distinctFull Article
structures
Yao Y.X.¶, Zhao Q.Y.¶, Tao Y.Q., Liu K.E., Cao T.Y., Chen Z.P., Liu C., Le W.D., Zhao J.,
Li D.*, Kang W.Y.*
J Biol Chem., 2024. (¶co-first author, *corresponding author) 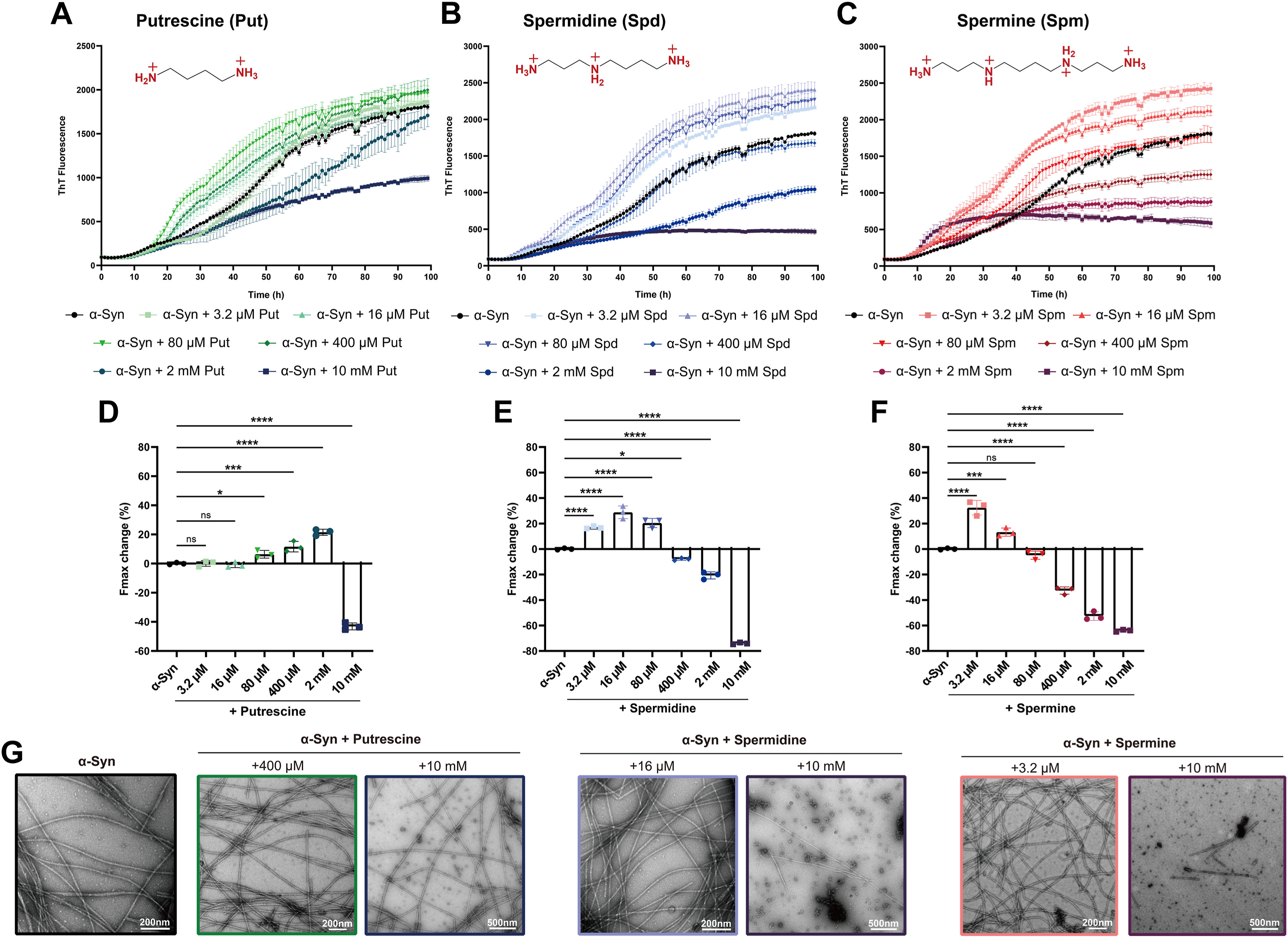
-

-
Full Article
Inhibitor Development for α-Synuclein Fibril’s Disordered Region to Alleviate
Parkinson's Disease Pathology
Zhang S.Q.¶, Xiang H.J.¶, Tao Y.Q.¶, Li J.¶, Zeng S.Y., Xu Q.H., Xiao H.N., Lv S.R.,
Song C.W., Cheng Y., Li M., Zhu Z.Y., Zhang S.N., Sun B., Li D., Xiang S.Q.*, Tan L.*,
Liu C.*
J Am Chem Soc., 2024. (¶co-first author, *corresponding author) 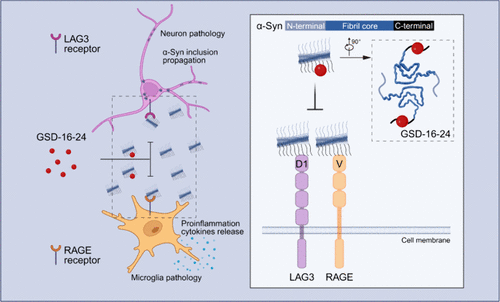
-

-
α-Synuclein amyloid fibril directly binds to LC3B and suppressesFull Article
SQSTM1/p62-mediated selective autophagy
Xu Q.H.¶, Wang H.L.¶, Yang R.N., Tao Y.Q., Wang Z.Y., Zhang S.N., Sun B.,
Li D., Lu B.X.*, Liu C.*
Cell Res., 2024. (¶co-first author, *corresponding author) 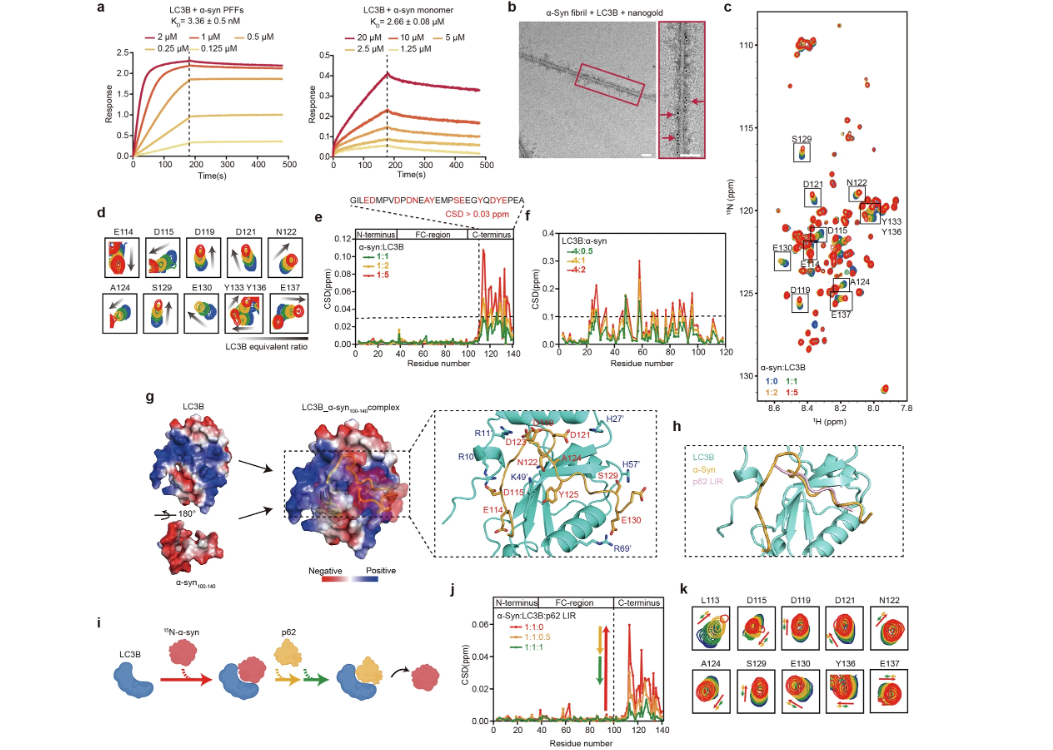
-

-
RPA transforms RNase H1 to a bidirectional exoribonuclease for processiveFull Article
RNA-DNA hybrid cleavage
Li Y.N.¶, Liu C.¶, Jia X.S., Bi L.L., Ren Z.Y., Zhao Y.L., Zhang X., Guo L.J., Bao Y.L.,
Liu C., Li W.*, Sun B.*
Nat Commun., 2024. (¶co-first author, *corresponding author) 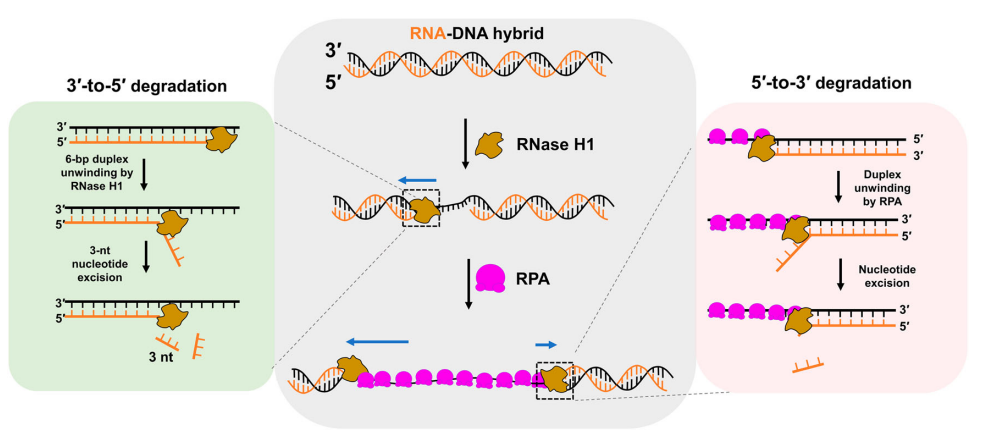
-
-
Pathological characteristics of axons and alterations of proteomic and lipidomicFull Article
profiles in midbrain dopaminergic neurodegeneration induced by
WDR45-deficiency
Wang P.P.¶, Shao Y.P., Murad A.N., Zhang J., Yang H.J., Yang Y.T., Kim K., Li S.,
Liu C., Cai H.B., Le W.D.*
Mol Neurodegener., 2024. (¶co-first author, *corresponding author) 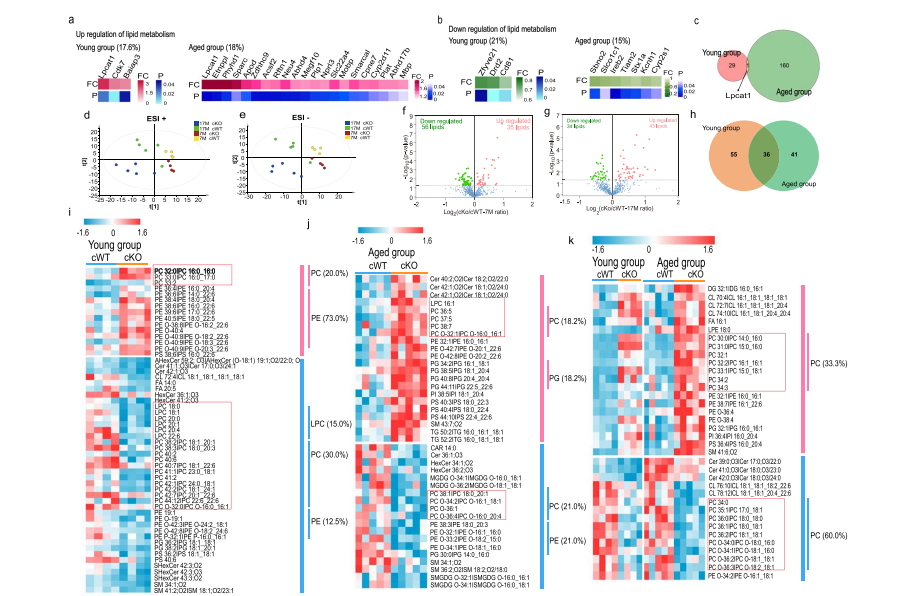
-

-
Binding adaptability of chemical ligands to polymorphic α-synuclein amyloid fibrilsFull Article
Liu K.E.¶, Tao Y.Q.¶, Zhao Q.Y., Xia W.C., Li X., Zhang S.Q., Yao Y.X., Xiang H.J., Han C., Tan L., Sun B., Li D., Li A., Liu C.*
Proc Natl Acad Sci U S A., 2024. (¶co-first author, *corresponding author) 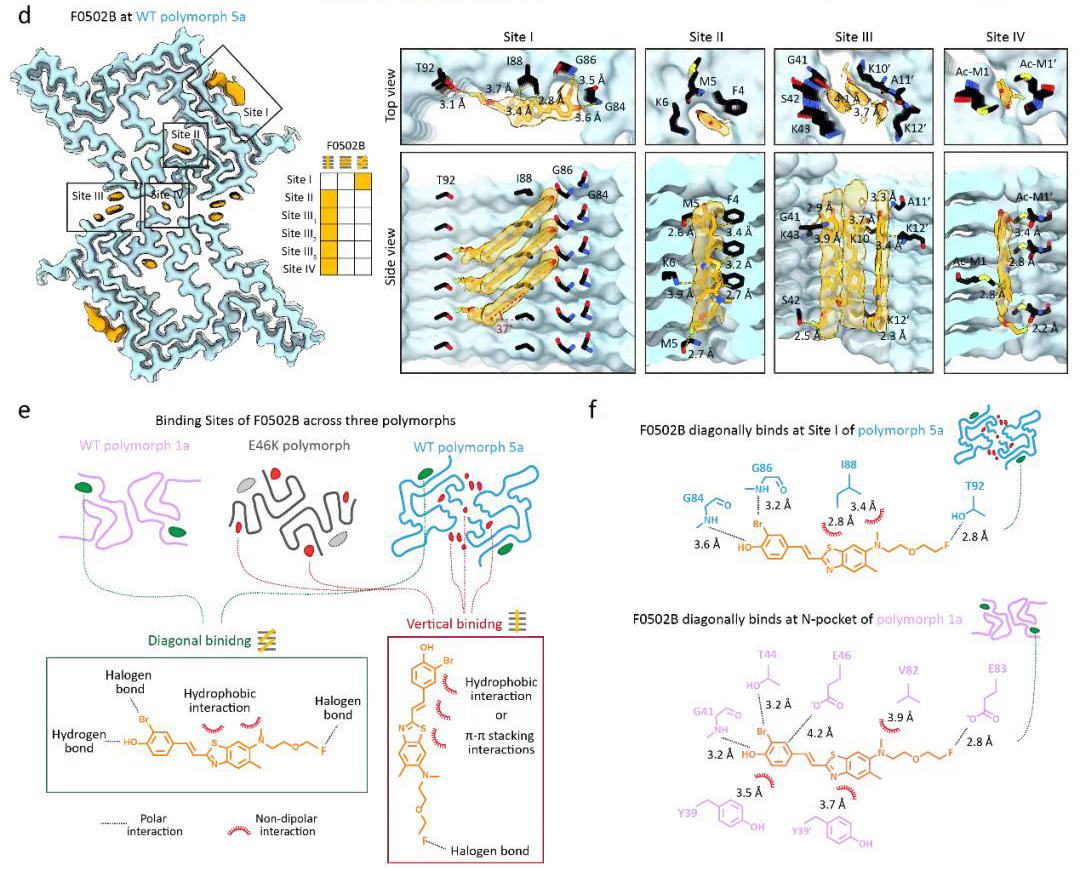
-
-
Fecal microbiota from patients with Parkinson's disease intensifies inflammationFull Article
and neurodegeneration in A53T mice
Yang H.J.¶, Shao Y.P., Hu Y.Y., Qian J., Wang P.P., Tian L.L., Ni Y., Li S.,
Murad Al-Nusaif., Liu C., Le W.D.*
CNS Neurosci Ther., 2024. (¶co-first author, *corresponding author) 
-

-
Ultrasensitive detection of aggregated α-synuclein using quiescent seedFull Article
amplification assay for the diagnosis of Parkinson's disease
Mao H.X.¶, Kuang Y.Y.¶, Feng D.¶, Chen X.¶, Lu L., Xia W.C., Gan T.T., Huang W.M.,
Guo W.Y., Yi H.C., Yang Y.R., Wu Z.H., Dai W., Sun H., Wu J.Y., Zhang R., Zhang S.Q.,
Lin X.L., Yong Y.X., Yang X.L., Li H.Y., Wu W.J., Huang X.Y., Bian Z.X., Wong.H.L.X.,
Wang X.L., Michael P., Ren Y., Liu C., Zou W.Q., Chen S.D., Xu P.Y.*
Transl Neurodegener., 2024. (¶co-first author, *corresponding author) 
-
-
VAMP2 chaperones α-synuclein in synaptic vesicle co-condensatesFull Article
Wang C.C.¶, Zhang K., Cai B., Jillian E.H., Kathryn E.C., Hu J.J., Zhao C.Y., Tian Z.Q., Hu X., Daniel Hall., Qiang J.L., Hou S.Q., Liu Z.Y., Gu J.G., Zhang Y.Y., Kim B.S., Jacqueline B., Fang Y.S., Liu C., Axel T.B., Li Dan.*, Diao J.J.*
Nat Cell Biol., 2024. (¶co-first author, *corresponding author) 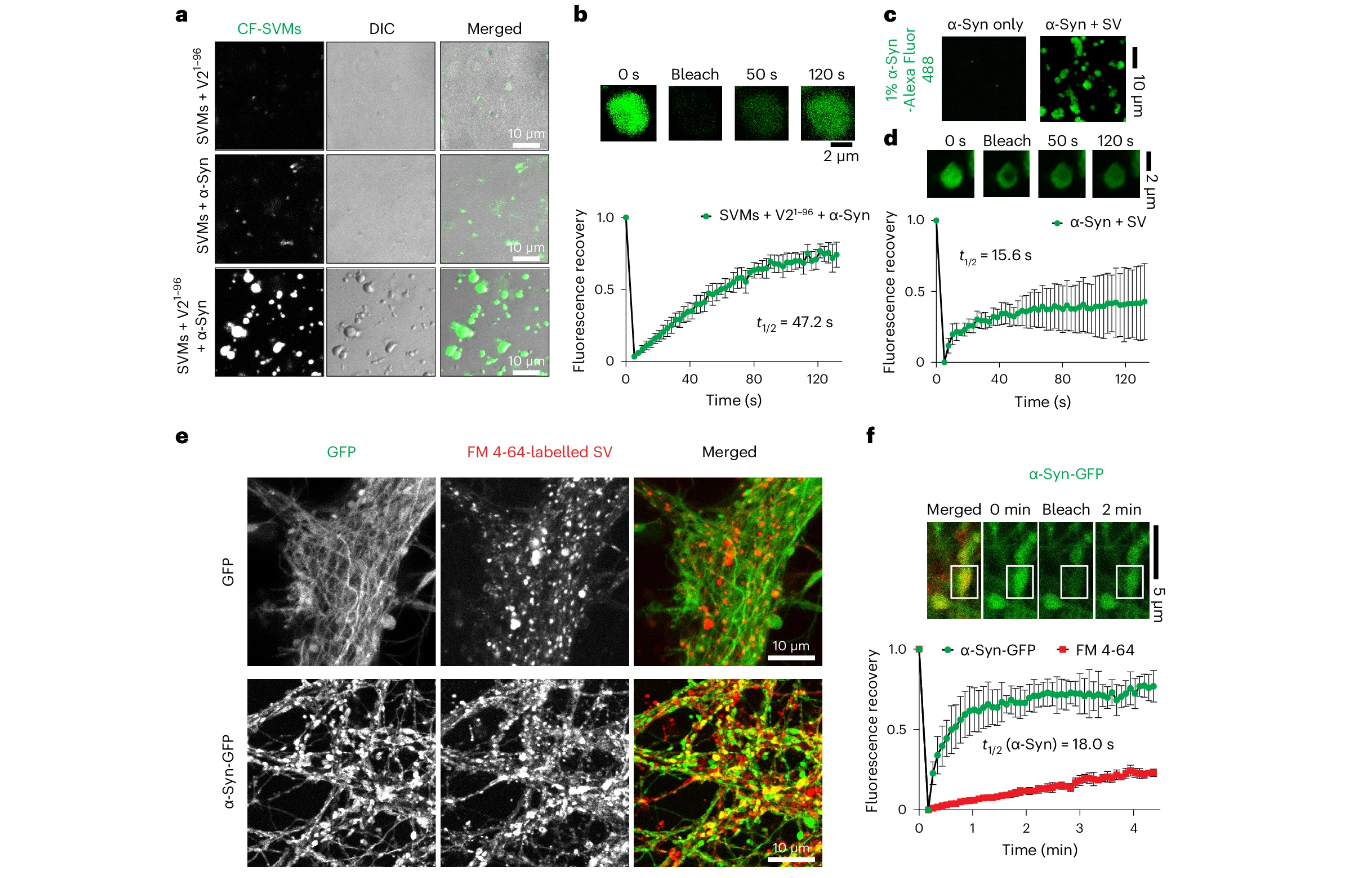
-

-
Phase-separated ParB enforces diverse DNA compaction modes and stabilizes the parS-centered partition complexFull Article
Zhao Y.L.¶, Guo L.J.¶, Hu J.J.¶, Ren Z.Y., Li Y.N., Hu M., Zhang X., Bi L.L., Li D.,
Ma H.H., Liu C.*, Sun Bo.*
Nucleic Acids Res., 2024. (¶co-first author, *corresponding author) 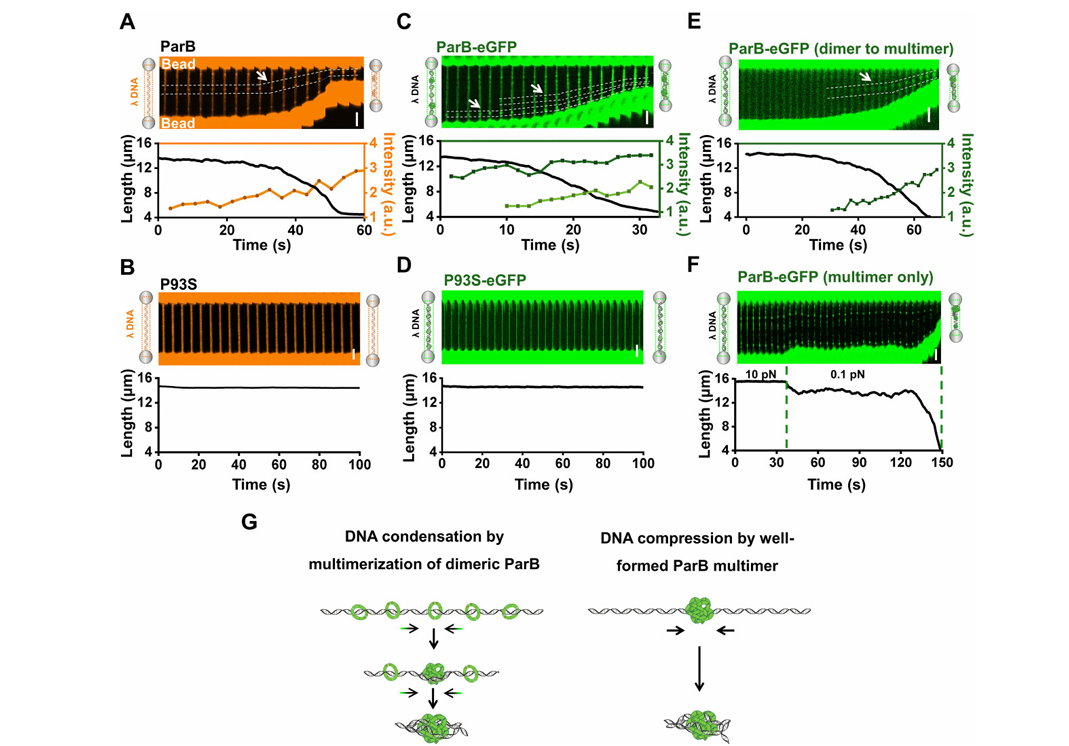
-

-
Hsp90α forms condensate engaging client proteins with RG motif repeatsFull Article
Hu J.J.*¶, Dong H., Li Y.C., Gu J.G., Yang L., Si C.F., Zhang Y.Y., Li T.T., Li D.*, Liu C.*
Chem Sci., 2024. (¶co-first author, *corresponding author) 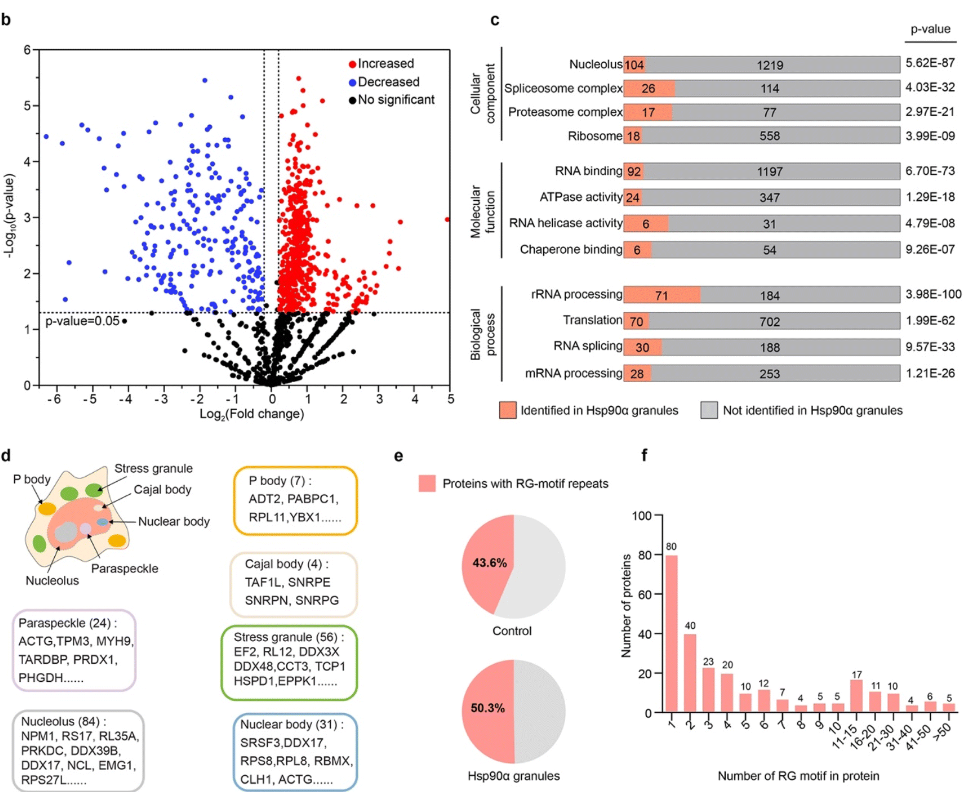
-

-
Full Article
Lysophosphatidylcholine binds α-synuclein and prevents its pathological
aggregation
Zhao C.Y.¶, Tu J.¶, Wang C.C.¶, Liu W.B., Gu J.G., Yin Y.D., Zhang S.N., Li D., Diao J.J.*, Zhu Z.J.*, Liu C.*
Natl Sci Rev., 2024. (¶co-first author, *corresponding author) 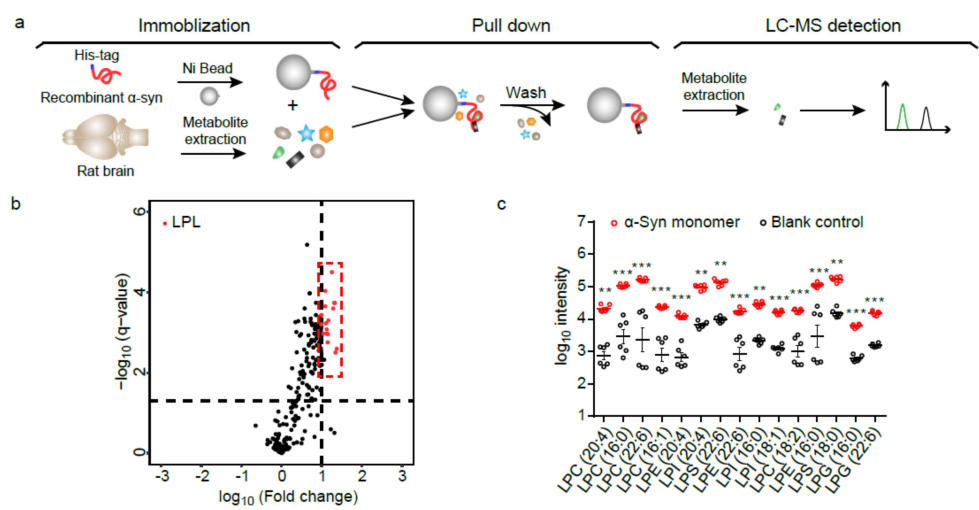
-

-
Aplp1 interacts with Lag3 to facilitate transmission of pathologic α-synucleinFull Article
Mao X.¶, Gu H.¶, Kim D.¶, Kimura Y., Wang N., Xu E., Kumbhar R., Ming X.,
Wang H., Chen C., Zhang S., Jia C., Liu Y., Bian H., Karuppagounder SS., Akkentli F.,
Chen Q., Jia L., Hwang H., Lee SH., Ke X., Chang M., Li A., Yang J., Rastegar C.,
Sriparna M., Ge P., Brahmachari S., Kim S., Zhang S., Shimoda Y., Saar M., Liu H.,
Kweon SH., Ying M., Workman CJ., Vignali DAA., Muller UC., Liu C., Ko HS.*,
Dawson VL.*, Dawson TM.*
Nat Commun., 2024. (¶co-first author, *corresponding author) 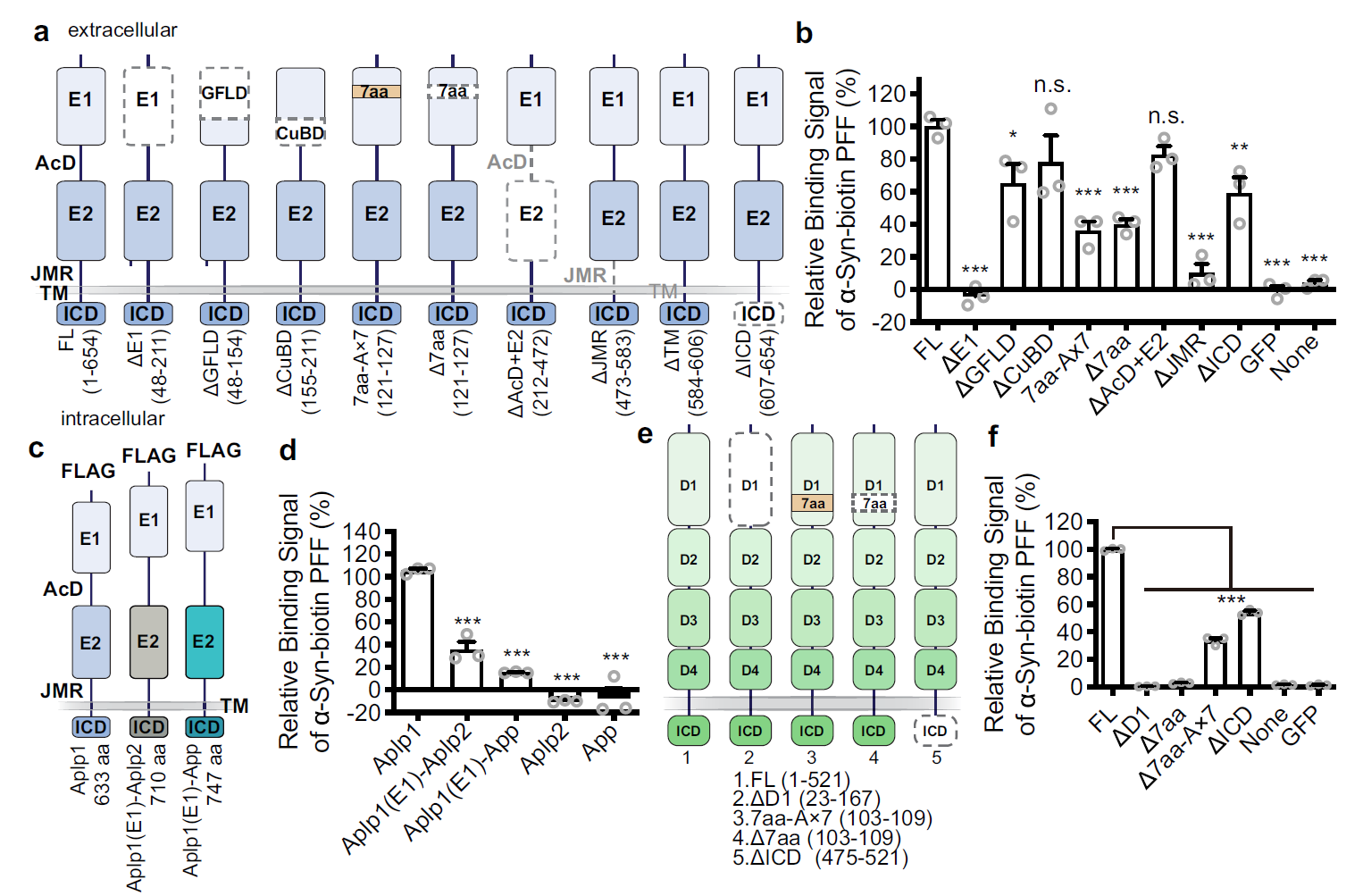
-
-
A Tau PET tracer PBB3 binds to TMEM106B amyloid fibril in brainFull Article
Zhao Q.Y.¶, Fan Y.¶, Zhao W.B., Ni Y., Tao Y.Q., Bian J., Xia W.C., Yu W.B., Fan Z.,
Liu C., Sun B., Le W.D., Li W.S., Wang J.*, Li D.*
Cell Discov., 2024. (¶co-first author, *corresponding author) 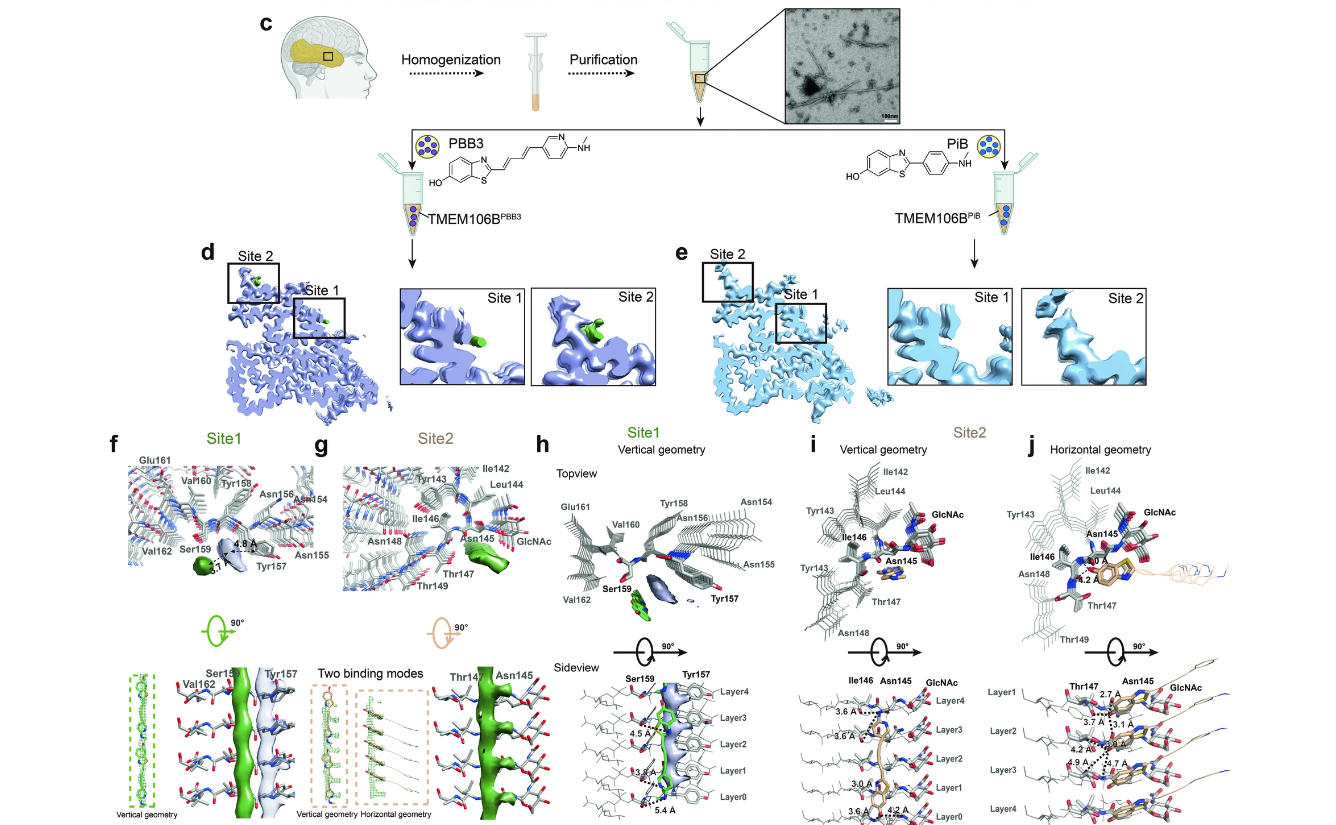
-

-
A phase-separated protein hub modulates resistance to Fusarium head blight in wheatFull Article
He Y.¶, Yang X.J.¶, Xia X.B.¶, Wang Y.H., Dong Y.F., Wu L., Jiang P., Zhang X., Jiang C., Ma H.X., Ma W.J., Liu C., Ryan W., Matthew R.T., Zhang Z.G., Li G.*
Cell Host Microbe., 2024. (¶co-first author, *corresponding author) 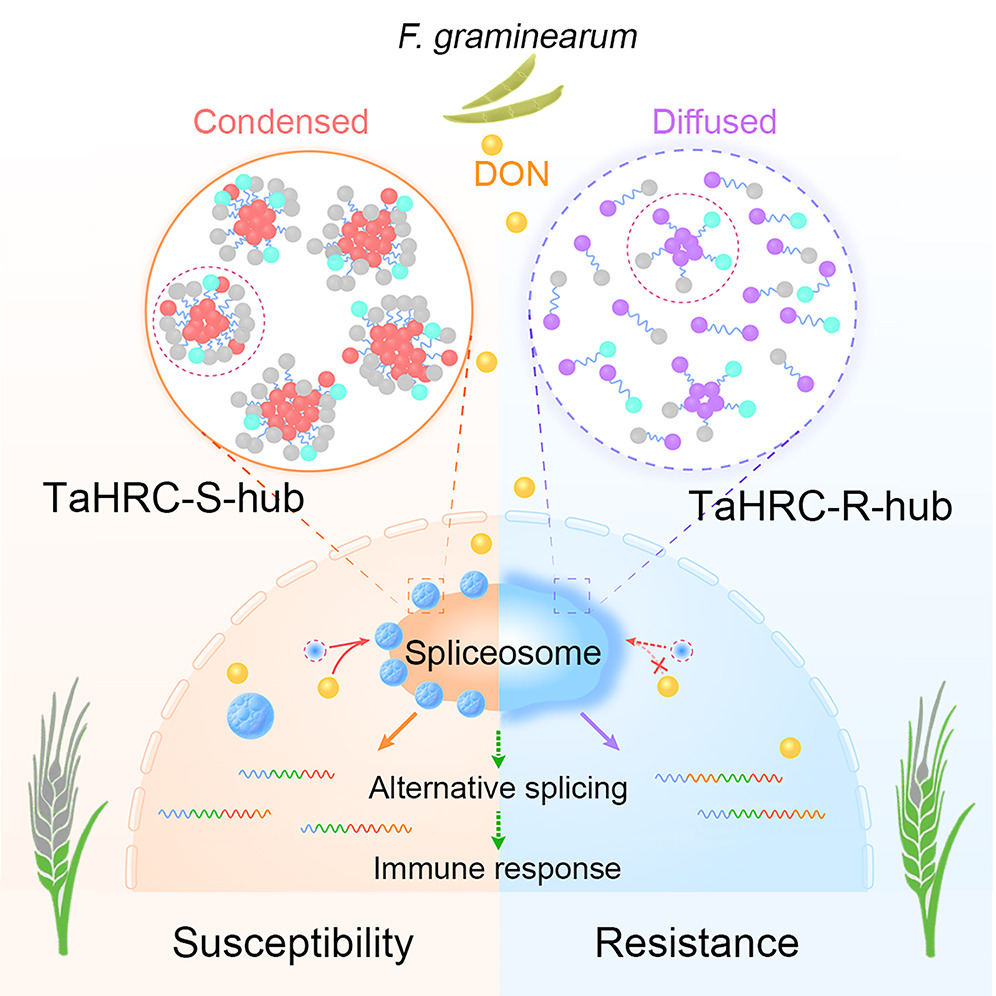
-

-
Phosphorylation and O-GlcNAcylation at the same α-synuclein site generate distinct fibril structuresFull Article
Hu J.J.¶, Xia W.C.¶, Zeng S.Y.¶, Lim Y.J.¶, Tao Y.Q., Sun Y.P., Zhao L., Wang H.S.,
Le W.D., Li D., Zhang S.N., Liu C.*, Li Y.M.*
Nat Commun., 2024. (¶co-first author, *corresponding author) 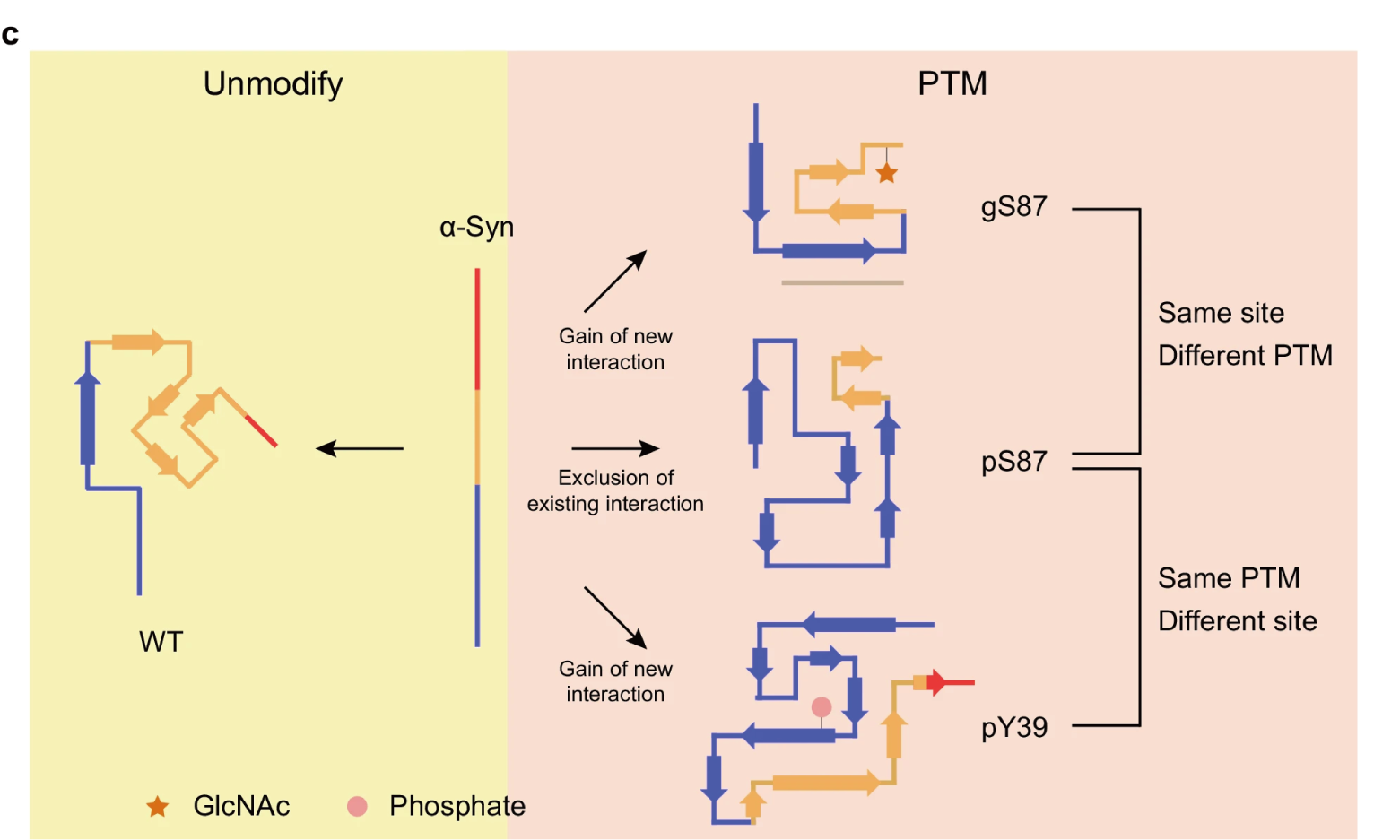
-

-
Halogen doped graphene quantum dots modulate TDP-43 phase separation and aggregation in the nucleusFull Article
Zhang H.¶, Guo H.Z., Li D.N., Zhang Y.L., Zhang S.Q., Kang W.Y., Liu C., Le W.D.,
Wang L.*, Li D.*, Dai B.*
Nat Commun., 2024. (¶co-first author, *corresponding author) 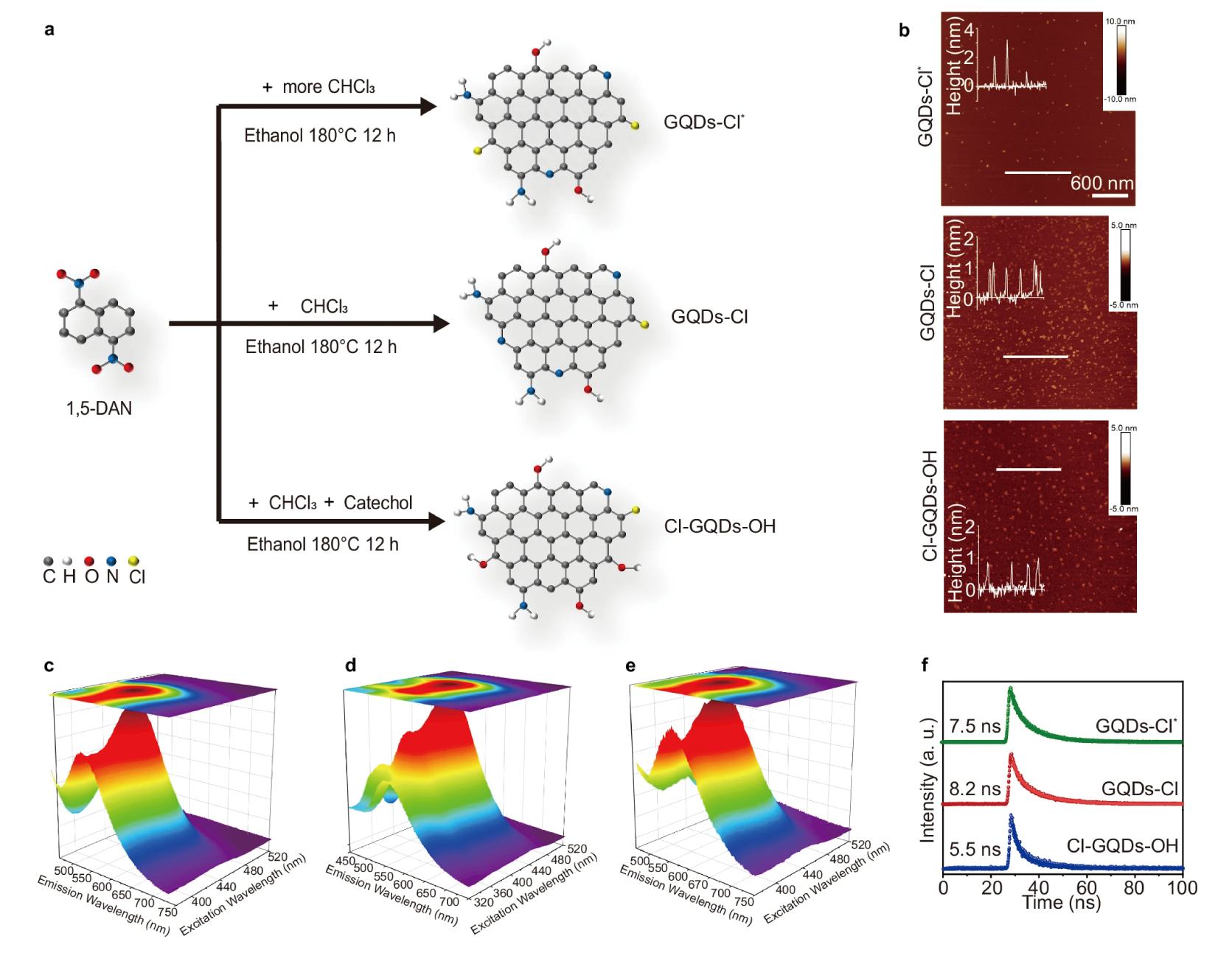
-

-
Machine learning predictor PSPire screens for phase-separating proteins lacking intrinsically disordered regionsFull Article
Hou S.¶, Hu J.J.¶, Yu Z.W., Li D, Liu C.*, Zhang Y.*
Nat Commun., 2024. (¶co-first author, *corresponding author) 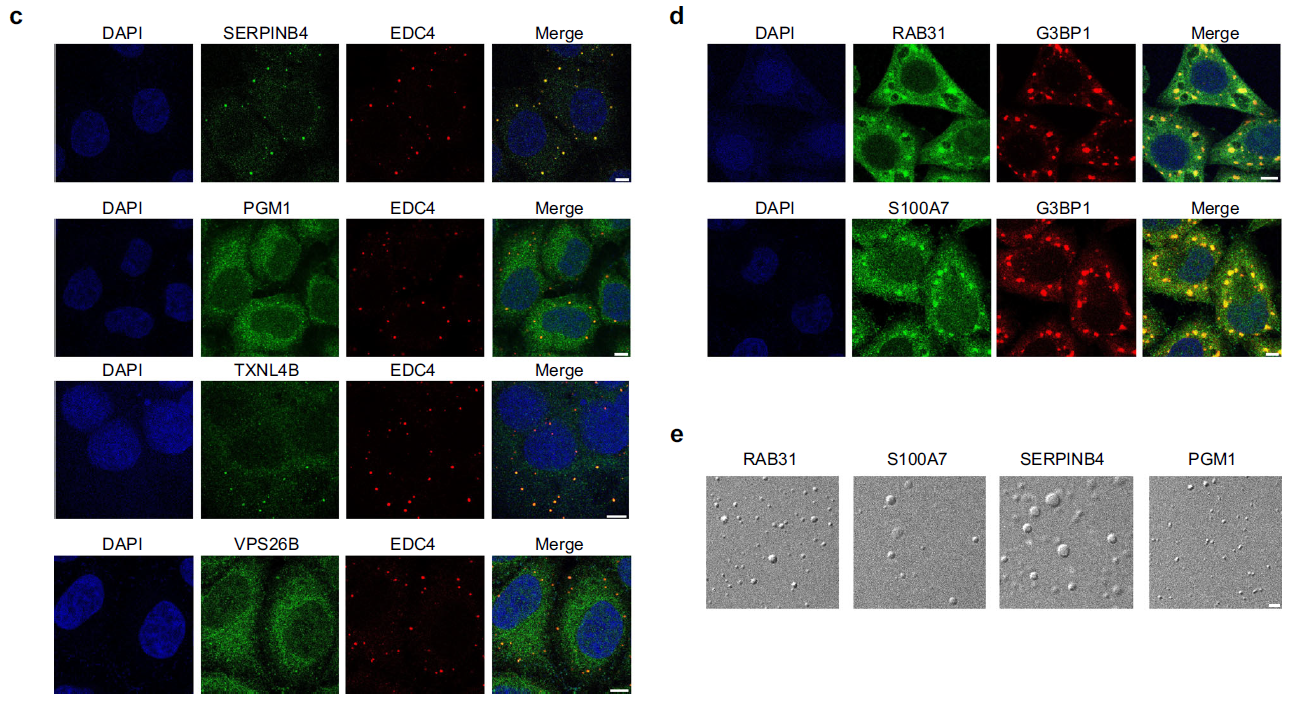
-
-
Cryo-EM structures reveal variant Tau amyloid fibrils between the rTg4510 mouse model and sporadic human tauopathiesFull Article
Zhao W.B.¶, Liu K.E.¶, Fan Y.¶, Zhao Q.Y., Tao Y.Q., Zhang M.W., Gan L.H., Yu W.B.,
Sun B., Li D., Liu C.*, Wang J.*
Cell Discov., 2024. (¶co-first author, *corresponding author) 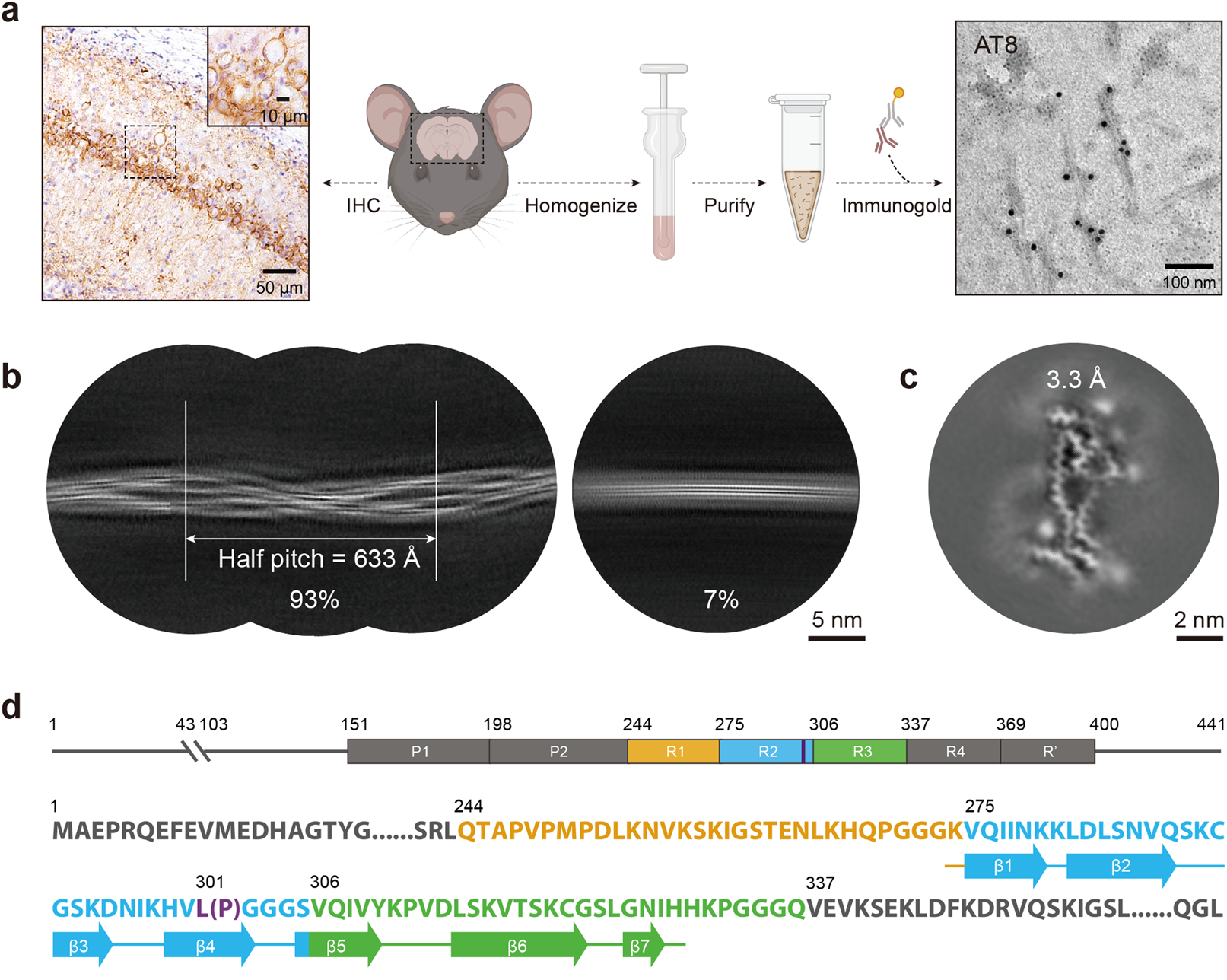
-

-
Phosphorylation-Regulated Dynamic Phase Separation of HIP-55 Protects Against Heart FailureFull Article
Jiang Y.Q.¶, Gu J.G.¶, Niu X.D.¶, Hu J.J., Zhang Y.Z., Li D., Tang Y.D., Liu C.*, Li Z.J.*
Circulation., 2024. (¶co-first author, *corresponding author) 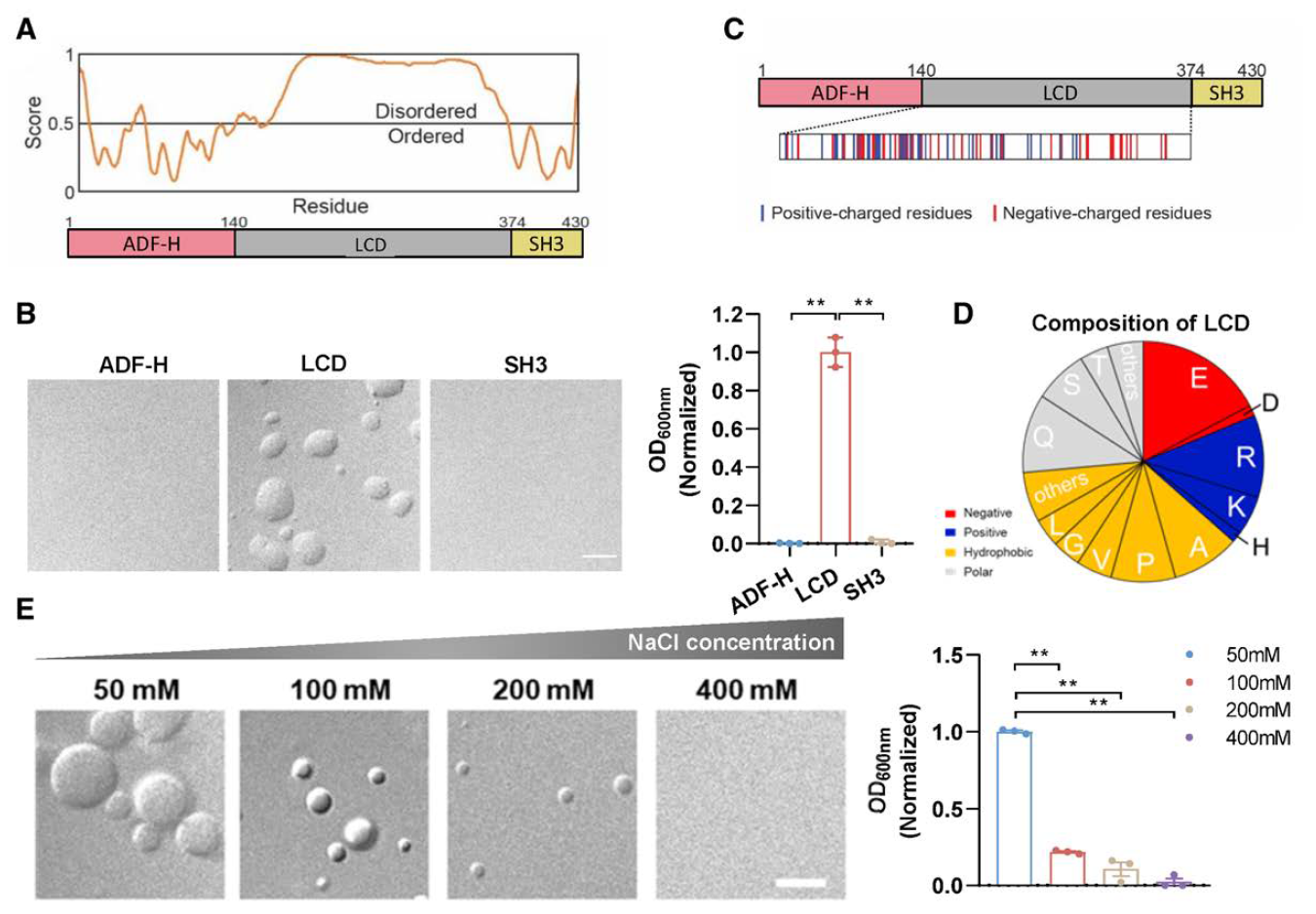
-

-
Advanced Techniques for Detecting Protein Misfolding and Aggregation inFull Article
Cellular Environments
Bai Y.L.¶, Zhang S.N.¶, Dong H.¶, Liu Y., Liu C.*, Zhang X.*
Chem Rev., 2023. (¶co-first author, *corresponding author) 
-

-
Molecular rules governing the structural polymorphism of amyloid fibrils inFull Article
neurodegenerative diseases
Li D.*, Liu C.*
Structure., 2023. (*corresponding author) 
-

-
Neutral lysophosphatidylcholine mediates α-synuclein-induced synaptic vesicleFull Article
clusteringLai Y.¶, Zhao C.Y.¶, Tian Z.Q.¶, Wang C.C.¶, Fan J.Q.¶, Hu X., Tu J., Li T.H.,
Proc Natl Acad Sci U S A., 2023. (¶co-first author, *corresponding author)
Leitz Jeremy., Pfuetzne R.A., Liu Z.T., Zhang S.N., Su Z.M., Burré J., Li D.,
Südhof Thomas.C., Zhu Z.J., Liu C.*, Brunger Axel.T.*, Diao J.J.* 
-

-
Creating an Amyloid 'Kaleidoscope' Using Short Iodinated PeptidesFull Article
Li D.N.¶, Ma Y.Y.¶, Xia W.C., Tao Y.Q., Zhang Y.L., Zhang H., Li D., Dai B.*, Liu C.*
Angew Chem Int Ed Engl., 2023. (¶co-first author, *corresponding author) 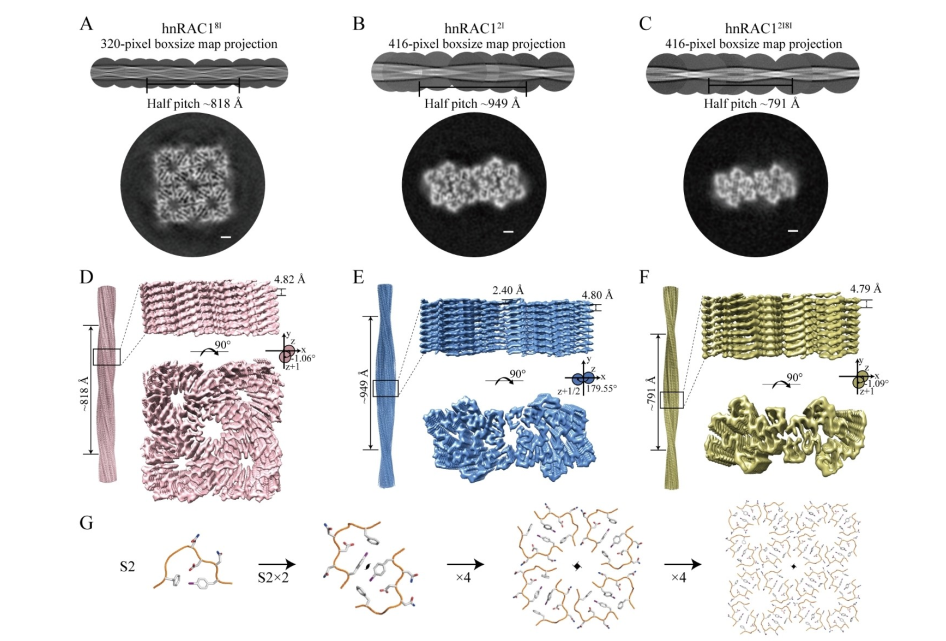
-

-
Engineering of antimicrobial peptide fibrils with feedback degradation ofFull Article
bacterial-secreted enzymes
Wang F.H.¶, Xia W.C.¶, Zhang M.M., Wu R.R., Song X.L., Hao Y., Feng Y.H.,
Zhang L.W., Li D., Kang W.Y., Liu C.*, Liu L.*
Chem Sci., 2023. (¶co-first author, *corresponding author) 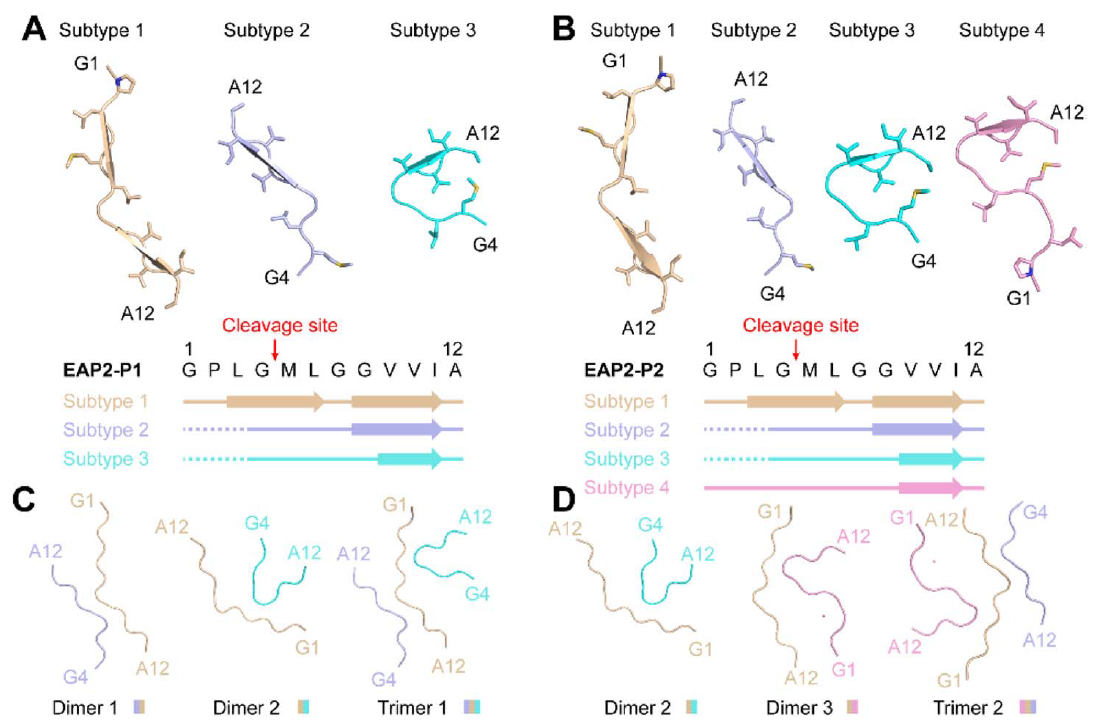
-

-
Rational design of functional amyloid fibrillar assembliesFull Article
Wang X.Y.¶, Zhang S.N.¶, Zhang J.C., Wang Y.M., Jiang X.Y., Tao Y.Q., Li D.,
Zhong C.*, Liu C.*
Chem Soc Rev., 2023. (¶co-first author, *corresponding author) 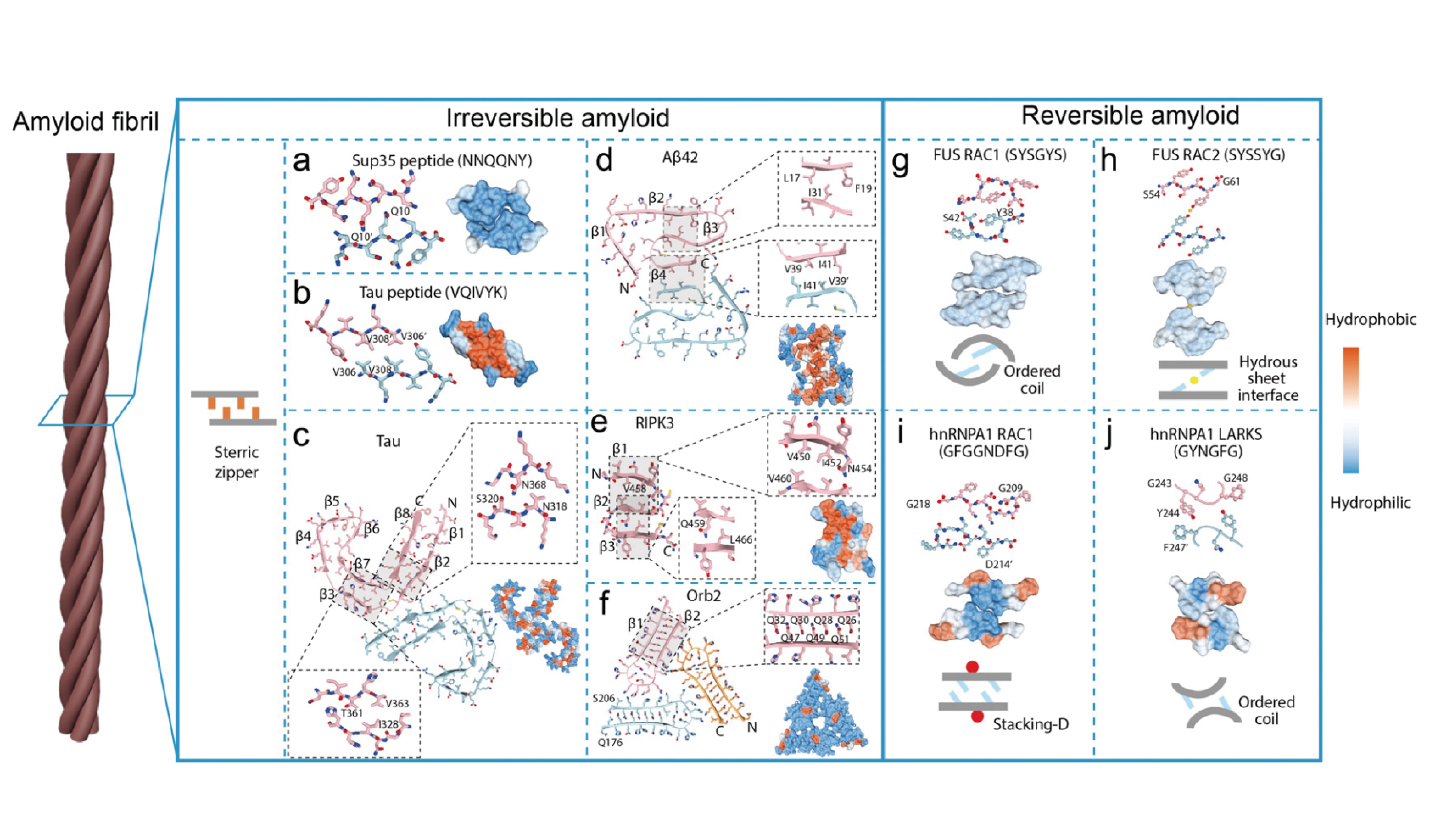
-
-
Development of an α-synuclein positron emission tomography tracer for imaging synucleinopathiesFull Article
Xiang J.¶, Tao Y.Q.¶, Xia Y.Y.¶, Luo S.L., Zhao Q.Y., Li B.W., Zhang X.Q., Sun Y.P., Xia W.C., Zhang M.M., Kang S.S., Ahn E.H., Liu X., Xie F., Guan Y.H., Yang J.J., Bu L,H., Wu S.X., Wang X.C., Cao X.B., Liu C., Zhang Z.T.*, Li D.*, Ye K.Q.*
Cell., 2023. (¶co-first author, *corresponding author) 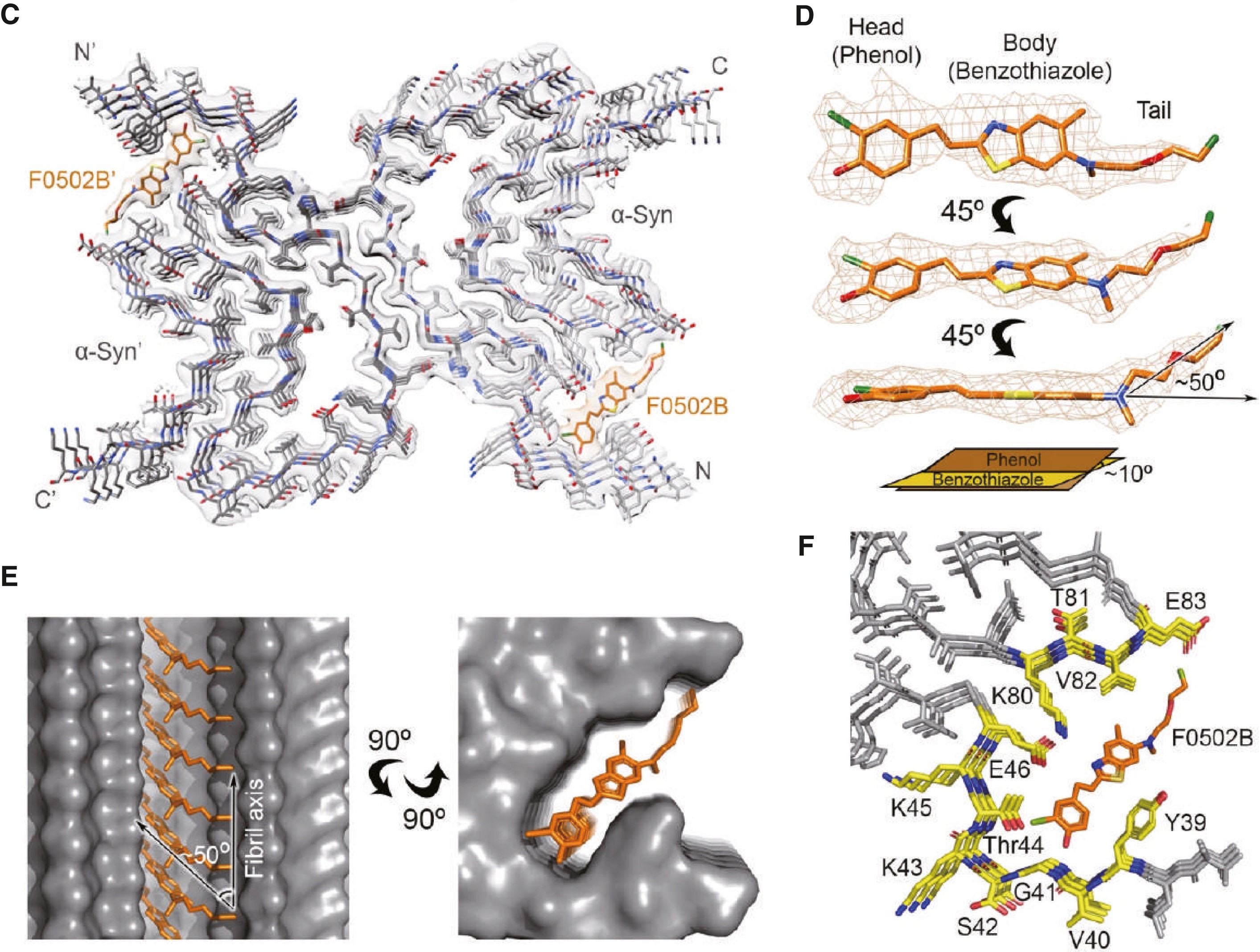
-

-
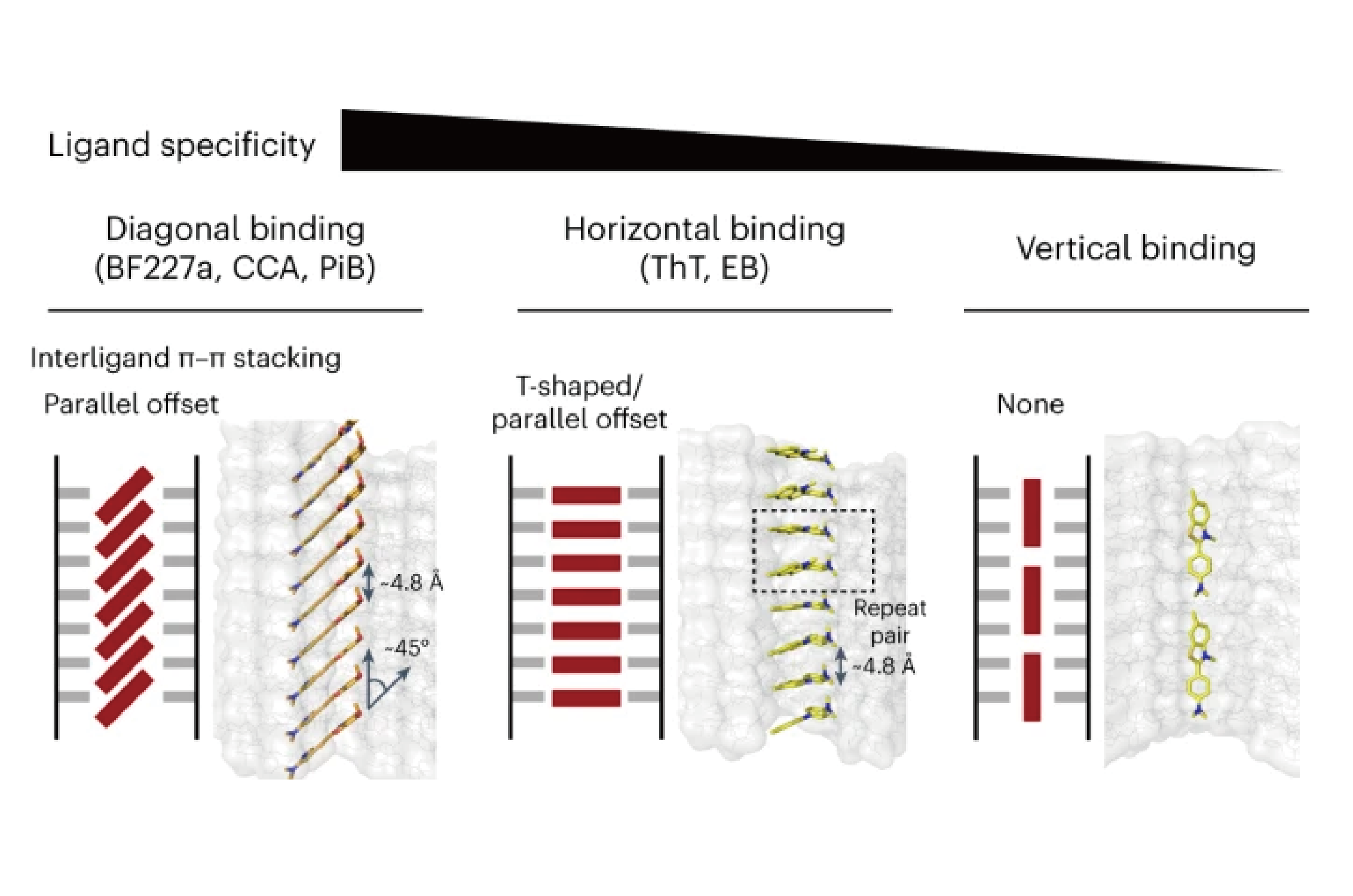
Full Article
Structural mechanism for specific binding of chemical compounds to amyloid
fibrilsTao Y.Q.¶, Xia W.C.¶, Zhao Q.Y.¶, Xiang H.J., Han C., Zhang S.Q., Gu W.,
Tang W.J, Li Y., Tan L., Li D.*, Liu C.*
Nat Chem Biol., 2023. (¶co-first author, *corresponding author)
-

-
β2-Microglobulin coaggregates with Aβ and contributes to amyloid pathologyFull Article
and cognitive deficits in Alzheimer's disease model mice
Zhao Y.N.¶, Zheng Q.Y., Hong Y.J., Gao Y., Hu J.J., Lang M.J., Zhang H.F., Zhou Y.,
Luo H., Zhang X., Sun H., Yan X.X., Huang T., Wang Y.J., Xu H., Liu C., Wang X.*
Nat Neurosci., 2023. (¶co-first author, *corresponding author) 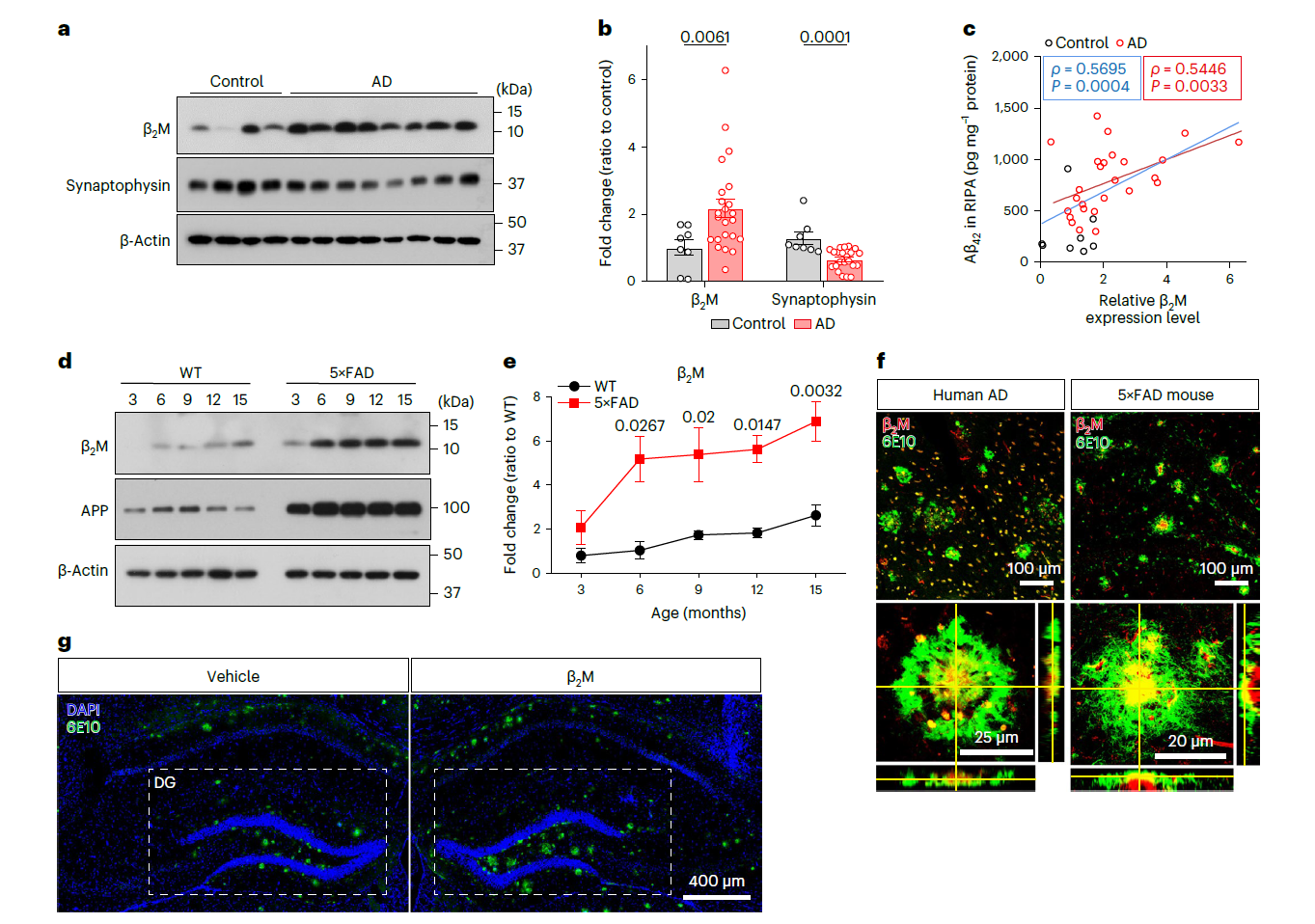
-

-
Targeting amyloid proteins for clinical diagnosis of neurodegenerative diseasesFull Article
Zhang S.Q.¶, Dong H.¶, Bian J.¶, Li D., Liu C.*
Fundamental Research., 2022. (¶co-first author, *corresponding author) 
-

-
Protein amyloid aggregate: Structure and functionFull Article
Xu Q.H.¶, Ma Y.Y.¶, Sun Y.P.¶, Li D., Zhang X., Liu C.*
Aggregate., 2023. (¶co-first author, *corresponding author) 
-

-
Modular characterization of SARS-CoV-2 nucleocapsid protein domain functionsFull Article
in nucleocapsid-like assembly
Wang Y.¶, Ling X.B.¶, Zhang C., Zou J., Luo B.N., Luo Y.B., Jia X.Y., Jia G.W.,
Zhang M.H., Hu J.C., Liu T., Wang Y.F.Y., Lu K.F., Li D., Ma J.B.*, Liu C.*, Su Z.M.*
Mol Biomed., 2023. (¶co-first author, *corresponding author) 
-
-
Graphene Quantum Dots Modulate Stress Granule Assembly and PreventFull Article
Abnormal Phase Transition of Fused in Sarcoma Protein
Zhang H.¶, Gu J.G., Zhang Y.L., Guo H.Z., Zhang S.N., Song J., Liu C., Wang L.*,
Li D.*, Dai B.*
ACS Nano., 2023. (¶co-first author, *corresponding author) 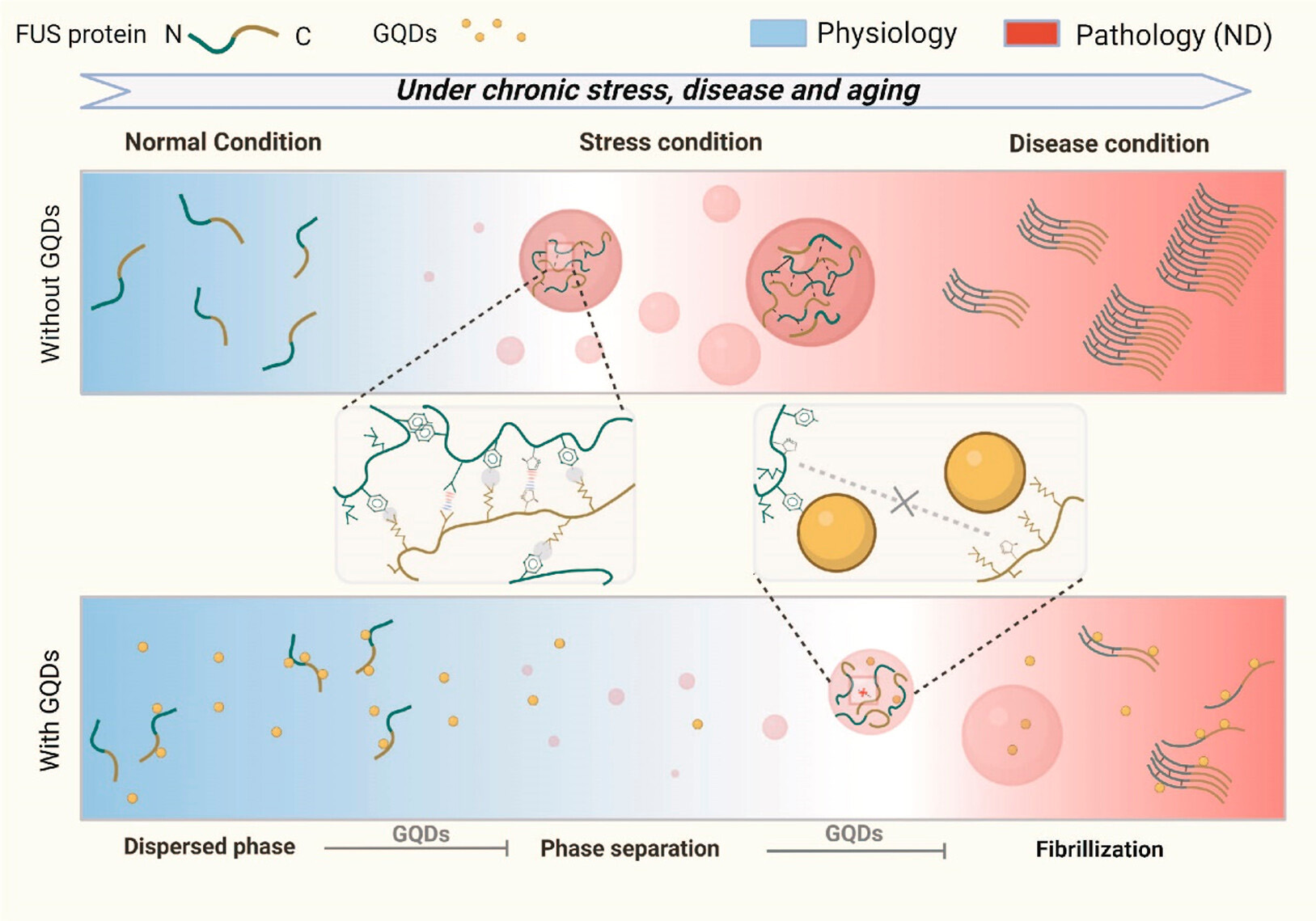
-
-
Emerging roles of O-glycosylation in regulating protein aggregation, phaseFull Article
separation, and functions
Li X.¶, Lv P.¶, Du Y.F.¶, Xing C.*, Liu C.*
Curr Opin Chem Biol., 2023. (¶co-first author, *corresponding author) 
-

-
Conformational Dynamics of an α-Synuclein Fibril upon Receptor BindingFull Article
Revealed by Insensitive Nuclei Enhanced by Polarization Transfer-Based
Solid-State Nuclear Magnetic Resonance and Cryo-Electron Microscopy
Zhang S.N.¶, Li J.¶, Xu Q.H.¶, Xia W.C., Tao Y.Q., Shi C.W., Li D., Xiang S.Q.*, Liu C.*
J Am Chem Soc., 2023. (¶co-first author, *corresponding author) 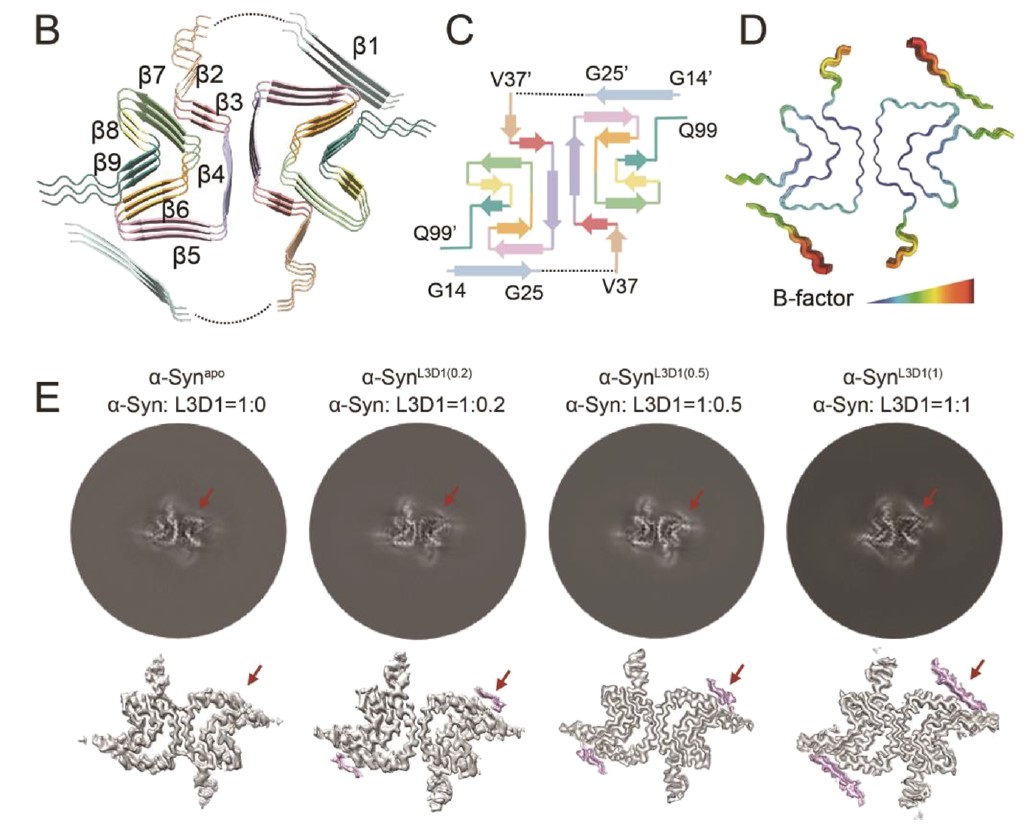
-

-
Cannabidivarin alleviates α-synuclein aggregation via DAF-16 in CaenorhabditisFull Article
elegans
Wang F.R.¶, Jin T.¶, Li H.Y.¶, Long H.F., Liu Y., Jin S., Lu Y.Y., Peng Y.H., Liu C.,
Zhao L.H.*, Wang X.H.*
FASEB J., 2023. (¶co-first author, *corresponding author) 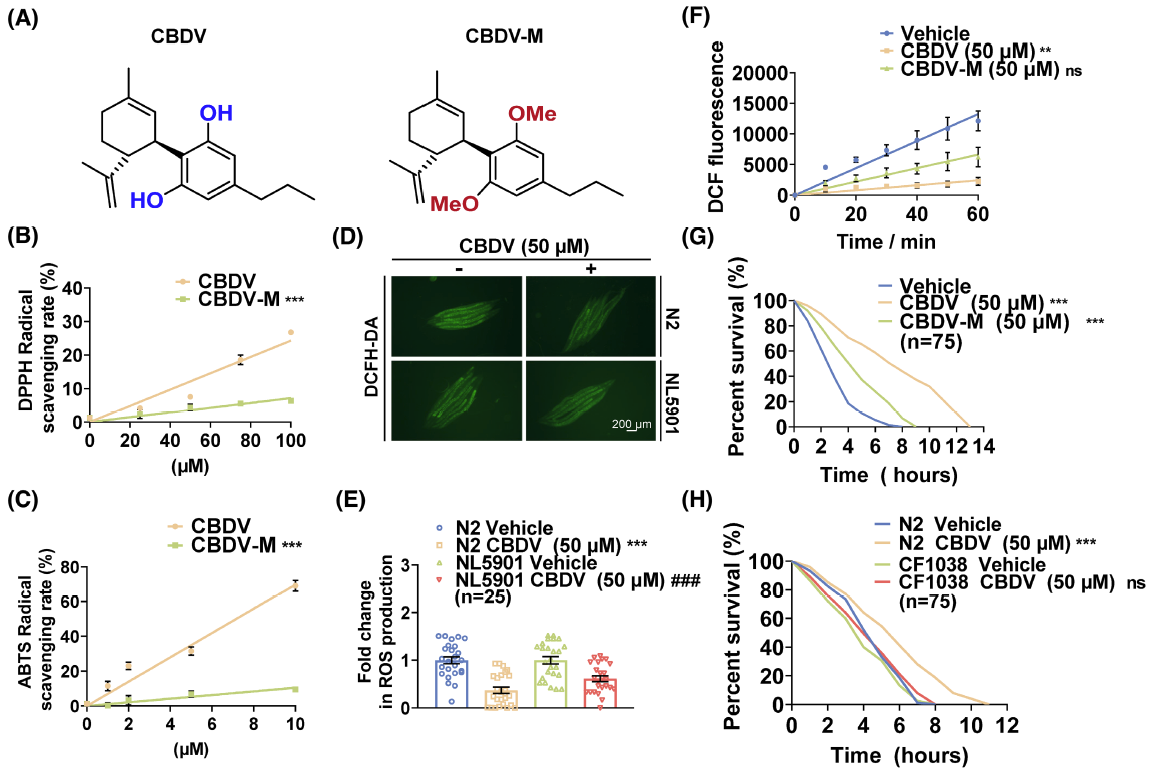
-

-
Intrastriatal injection of Parkinson's disease intestine and vagus lysates initiatesFull Article
α-synucleinopathy in rat brain
Yang Z.F.¶, Wang Y.¶, Wei M., Li S., Jia C.C., Cheng C., Nusaif M., Zhang J., Liu C.,
Le W.D.*
Cell Death Dis., 2023. (¶co-first author, *corresponding author) 
-

-
Conformational change of α-synuclein fibrils in cerebrospinal fluid from different clinical phases of Parkinson’s diseaseFull Article
Fan Y.¶, Sun Y.P.¶, Yu W.B., Tao Y.Q., Xia W.C., Liu Y.Q., Zhao Y.Q., Tang Y.L., Sun Y.M., Liu F.T., Cao Q., Wu J.J., Liu C., Wang J.*, Li D.*
Structure., 2022. (¶co-first author, *corresponding author) 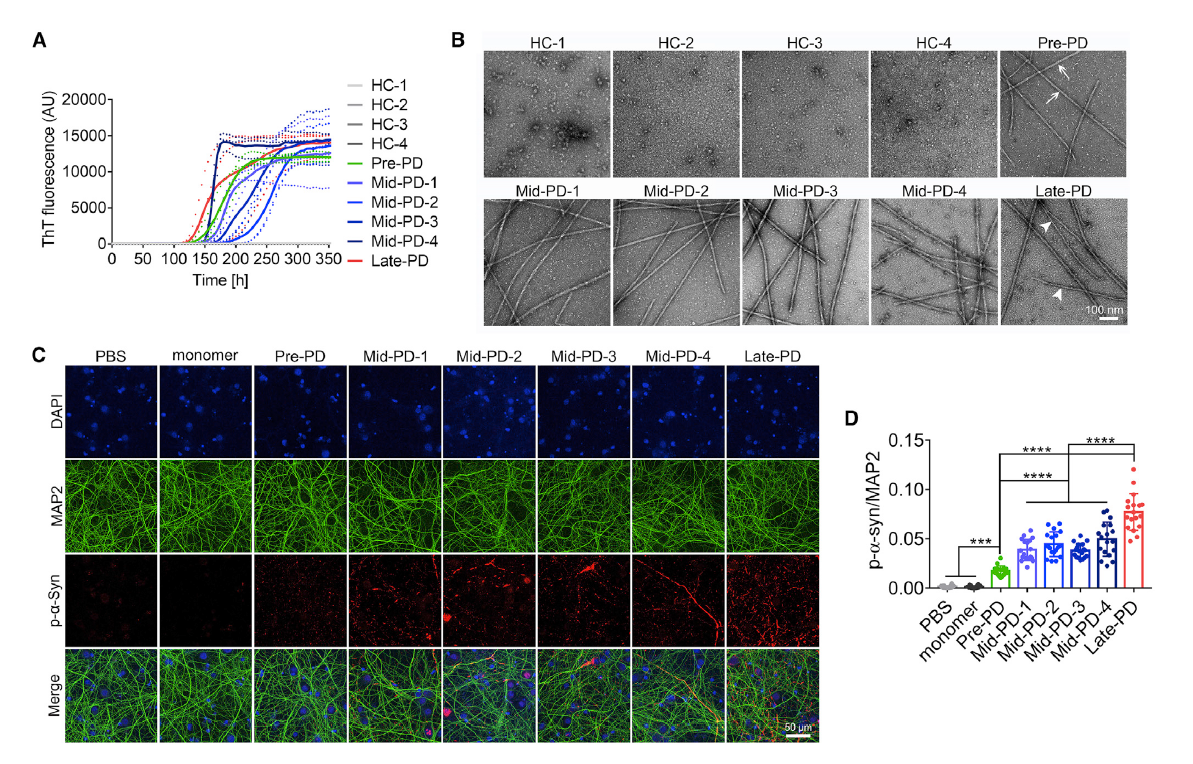
-

-
Subtle change of fibrillation condition leads to substantial alteration ofFull Article
recombinant Tau fibril structure
Li X.¶, Zhang S.Q.¶, Liu Z.T.¶, Tao Y.Q., Xia W.C., Sun Y.P., Liu C., Le W.D., Sun B.,
Li D.*
iScience., 2022. (¶co-first author, *corresponding author) 
-
-
Activating NO-sGC crosstalk in the mouse vascular niche promotes vascularFull Article
integrity and mitigates acute lung injury
He H.¶, Yang W.¶, Su N.¶, Zhang C.K., Dai J.N., Han F., Singhai M., Bai W.J., Zhu X.L., Zhu J., Liu Z., Xia W.C., Liu X.T., Zhang C.H., Jiang K., Huang W.H., Chen D.,
Wang Z.Y., He X.Y., Kirchhoff F., Li Z.Y., Liu C., Huan J.N., Wang X.H., Wei W.,
Wang J., Augustin H.G., Hu J.H.*
J Exp Med., 2023. (¶co-first author, *corresponding author) 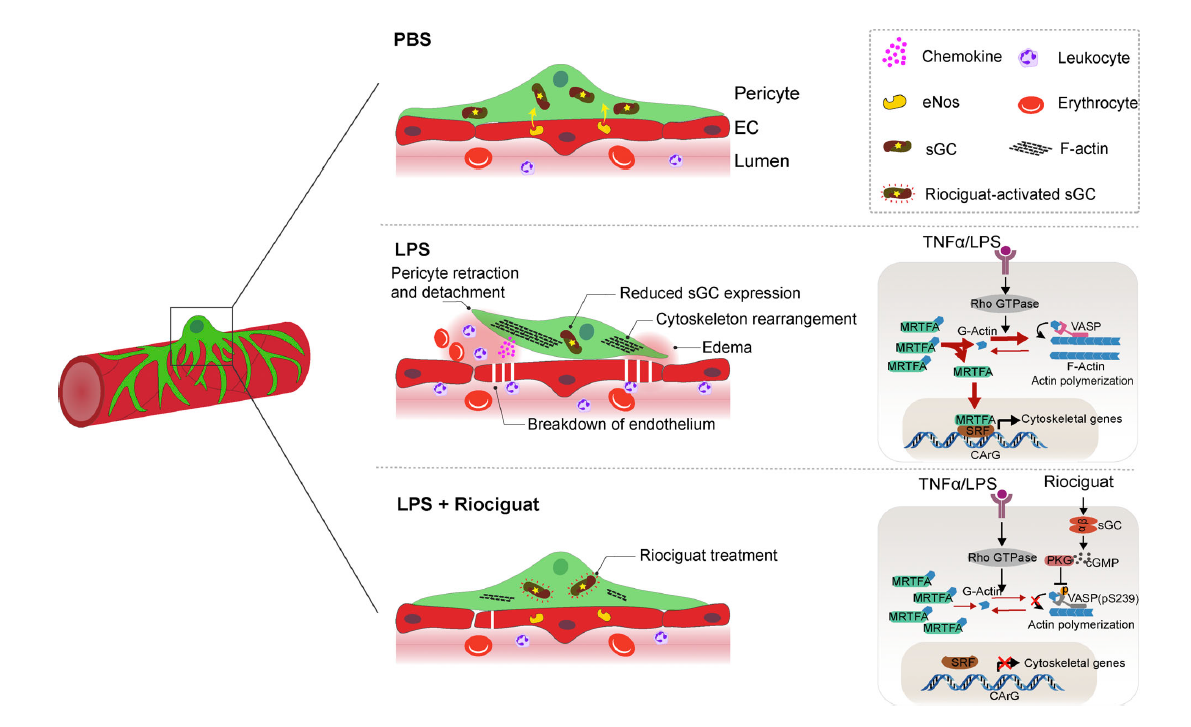
-

-
Global profiling of arginine dimethylation in regulating protein phase separationFull Article
by a steric effect-based chemical-enrichment method
Wang Q.¶, Li Z.X.¶, Zhang S.Q.¶, Li Y.C., Wang Y., Fang Z., Ma Y.N., Liu Z., Li D.,
Liu C.*, Ye M.L.*
Proc. Natl. Acad. Sci. U S A., 2022. (¶co-first author, *corresponding author) 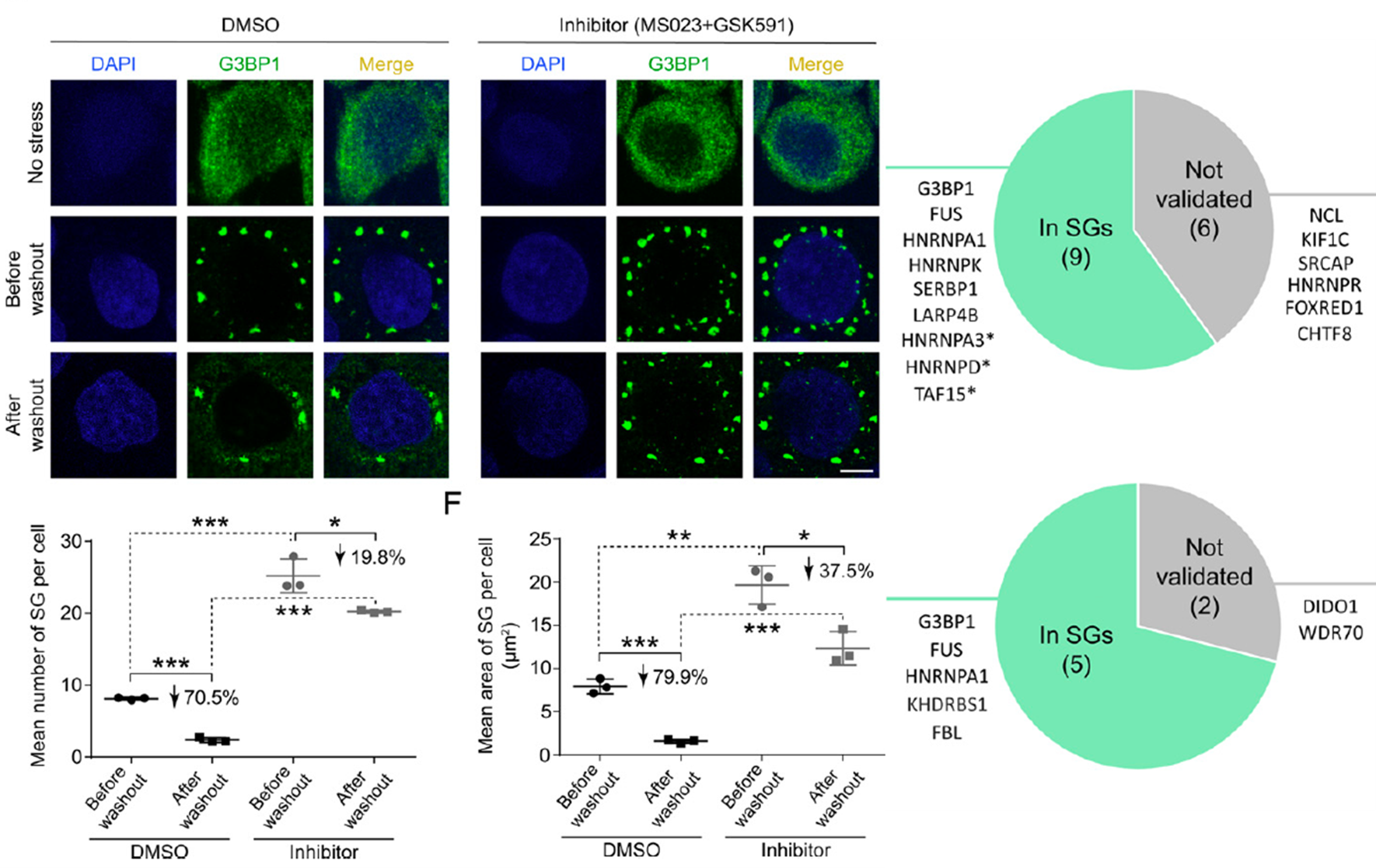
-

-
A High-Throughput Method to Profile Protein Liquid-Liquid Phase SeparationFull Article
Li Y.C., Gu J.G., Liu C., Li D.*
Methods in Molecular Biology., 2022 (*corresponding author) 
-

-
Interaction of RAGE with α-synuclein fibrils mediates inflammatory response ofFull Article
microglia
Long H.F.¶, Zhang S.N.¶, Zeng S.Y., Tong Y.L., Liu J., Liu C.*, Li D.*
Cell Rep., 2022. (¶co-first author, *corresponding author) 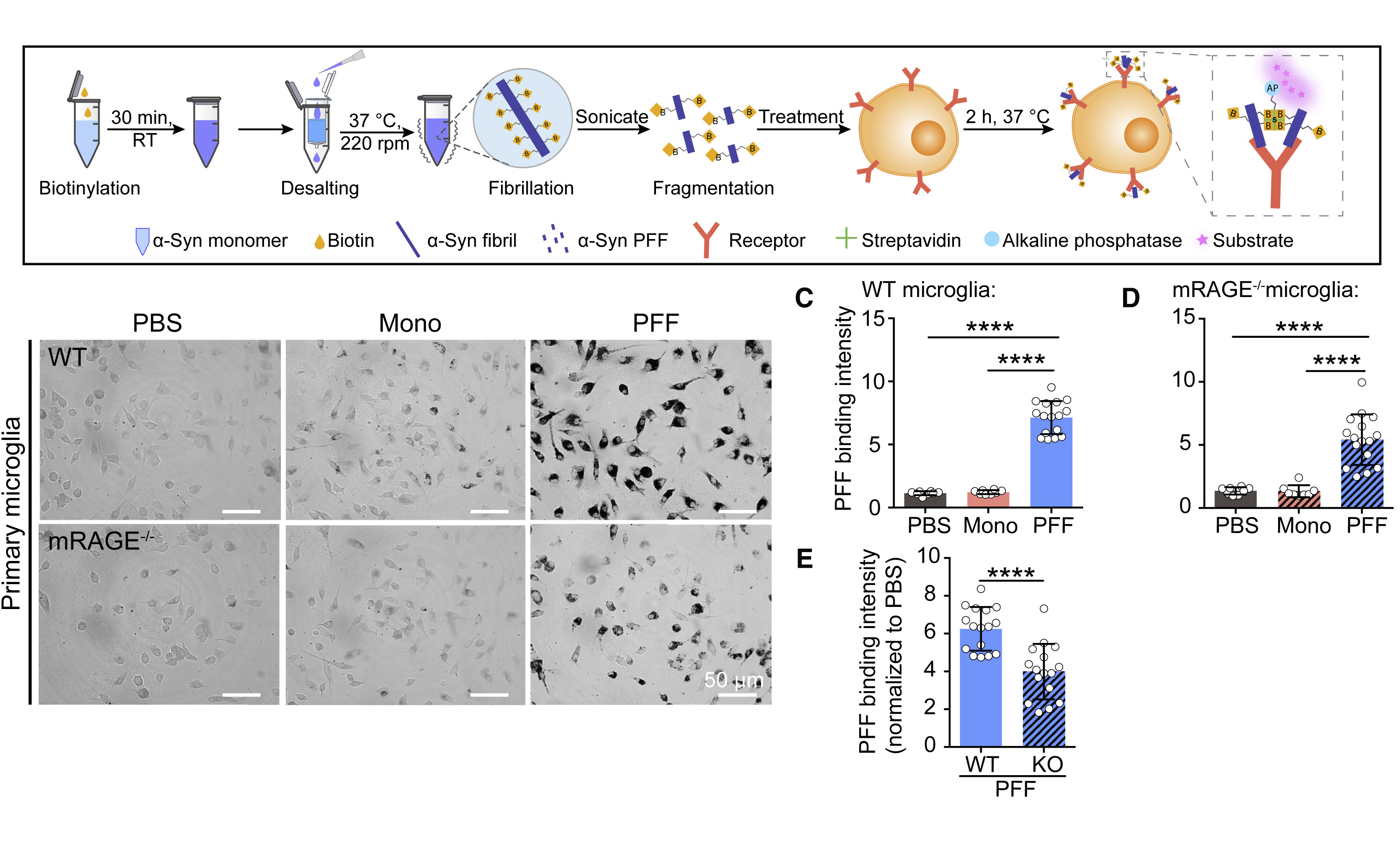
-

-
Non-ergodicity of a globular protein extending beyond its functional timescaleFull Article
Li J.¶, Xie J.F., Godec A., Weninger K.R., Liu C., Smith J.C., Hong L.*
Chem Sci., 2022. (¶co-first author, *corresponding author) 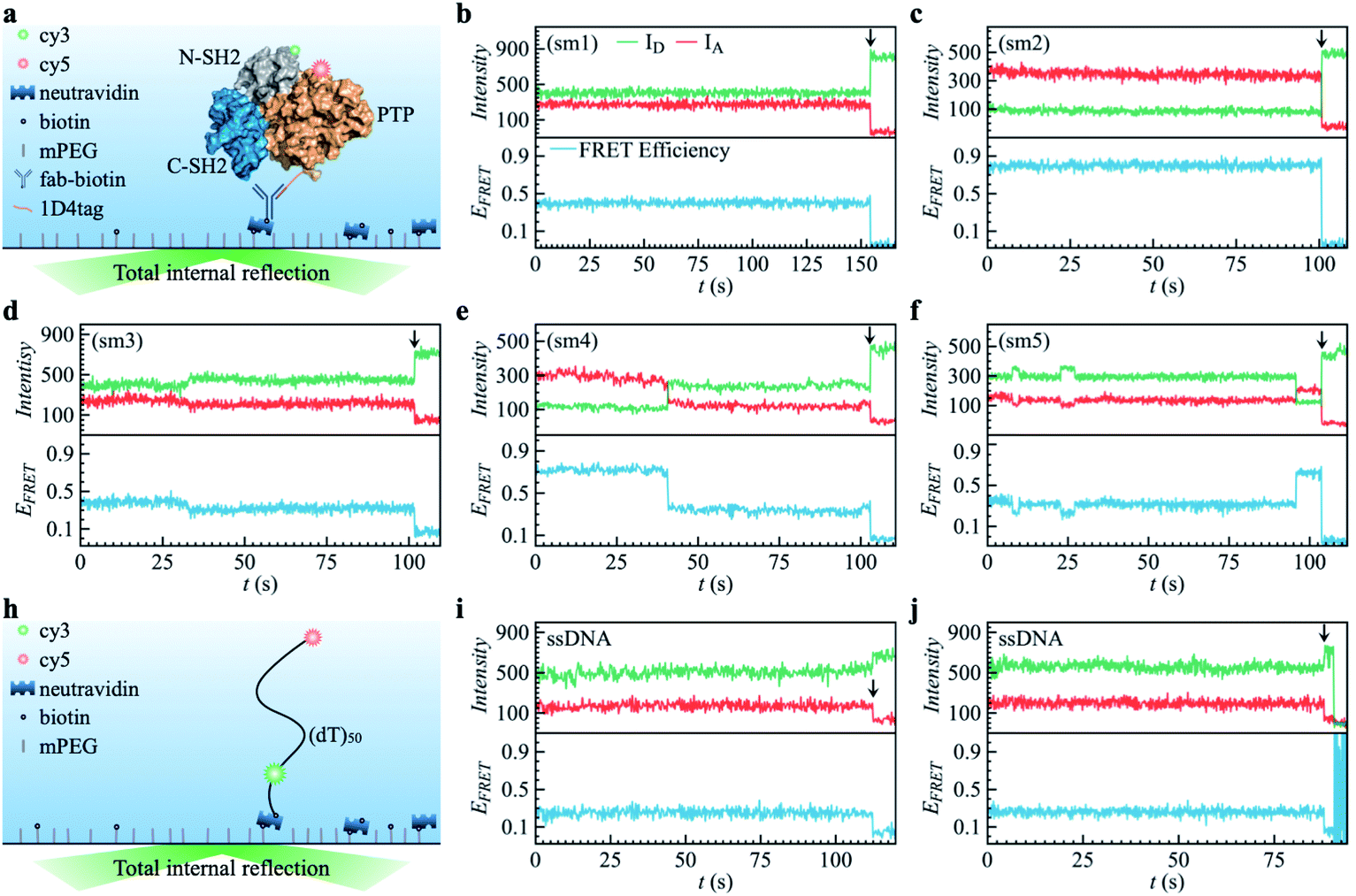
-
-
Heat-shock chaperone HSPB1 regulates cytoplasmic TDP-43 phase separationFull Article
and liquid-to-gel transition
Lu S.¶, Hu J.J., Arogundade O.A., Goginashvili A., Vazquez-Sanchez S., Diedrich J.K., Gu J.G., Blum J., Oung S., Ye Q.Z., Yu H.Y., Ravits J., Liu C., Yates J.R.,
Cleveland D.W.*
Nat Cell Biol. 2022. (¶co-first author, *corresponding author) 
-

-
Specific binding of Hsp27 and phosphorylated Tau mitigates abnormal TauFull Article
aggregation-induced pathology
Zhang S.N.¶, Zhu Y.¶, Lu J.X.¶, Liu Z.Y., Lobato A.G., Zeng W., Liu J.Q., Zeng S.Y.,
Liu C., Liu J., He Z.H., Zhai R.G.*, Li D.*
Elife., 2022. (¶co-first author, *corresponding author) 
-

-
Bloom Syndrome Helicase Compresses Single‐Stranded DNA intoFull Article
Phase‐Separated Condensates
Wang T.¶, Hu J.J.¶, Li Y.N., Bi L.L., Guo X.S., Zhang X., Li D., Hou X.M., Modesti M.,
Xi X.G., Liu C.*, Sun B.*
Angew Chem Int Ed Engl., 2022. (¶co-first author, *corresponding author) 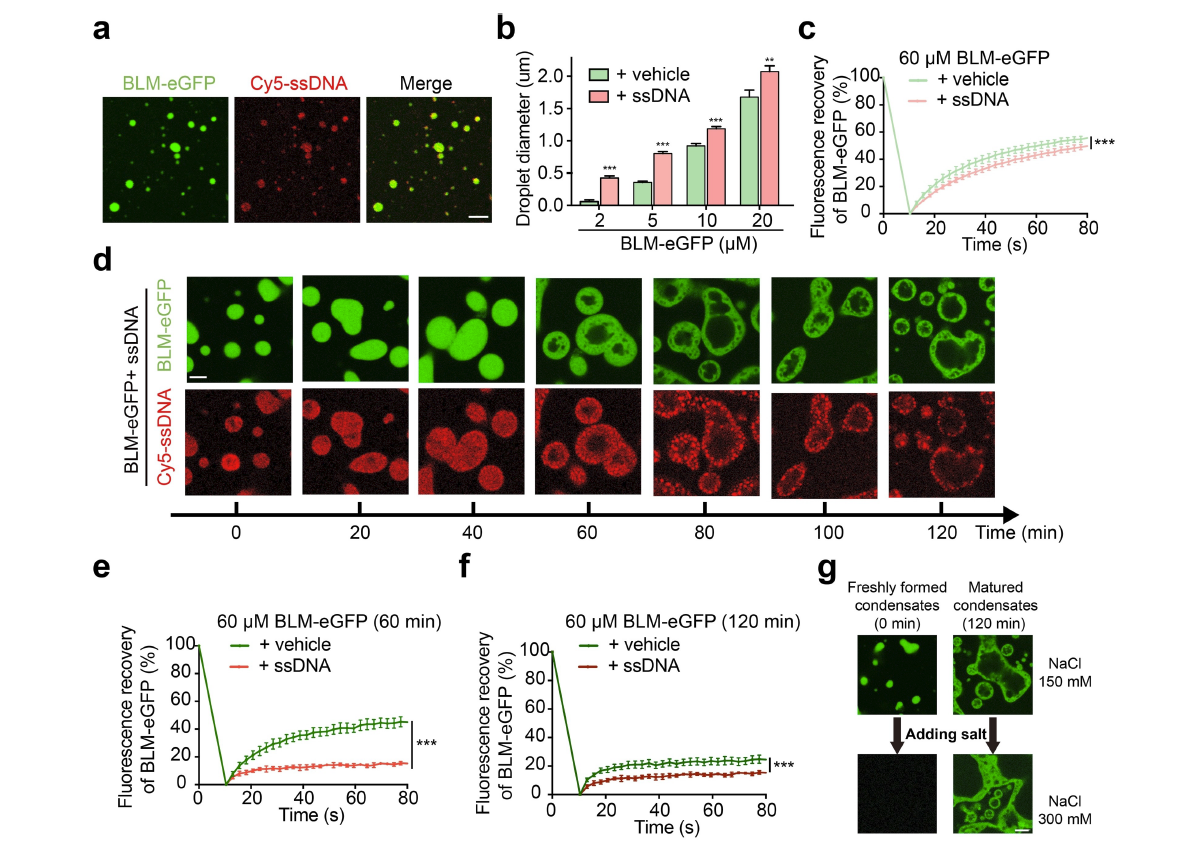
-

-
Stochastically multimerized ParB orchestrates DNA assembly as unveiled byFull Article
single-molecule analysis
Guo J.L.¶, Zhao Y.L.¶, Zhang Q., Feng Y., Zhang X., Wang T., Liu C., Ma H.H., Sun B.*
Nucleic Acids Res., 2022. (¶co-first author, *corresponding author) 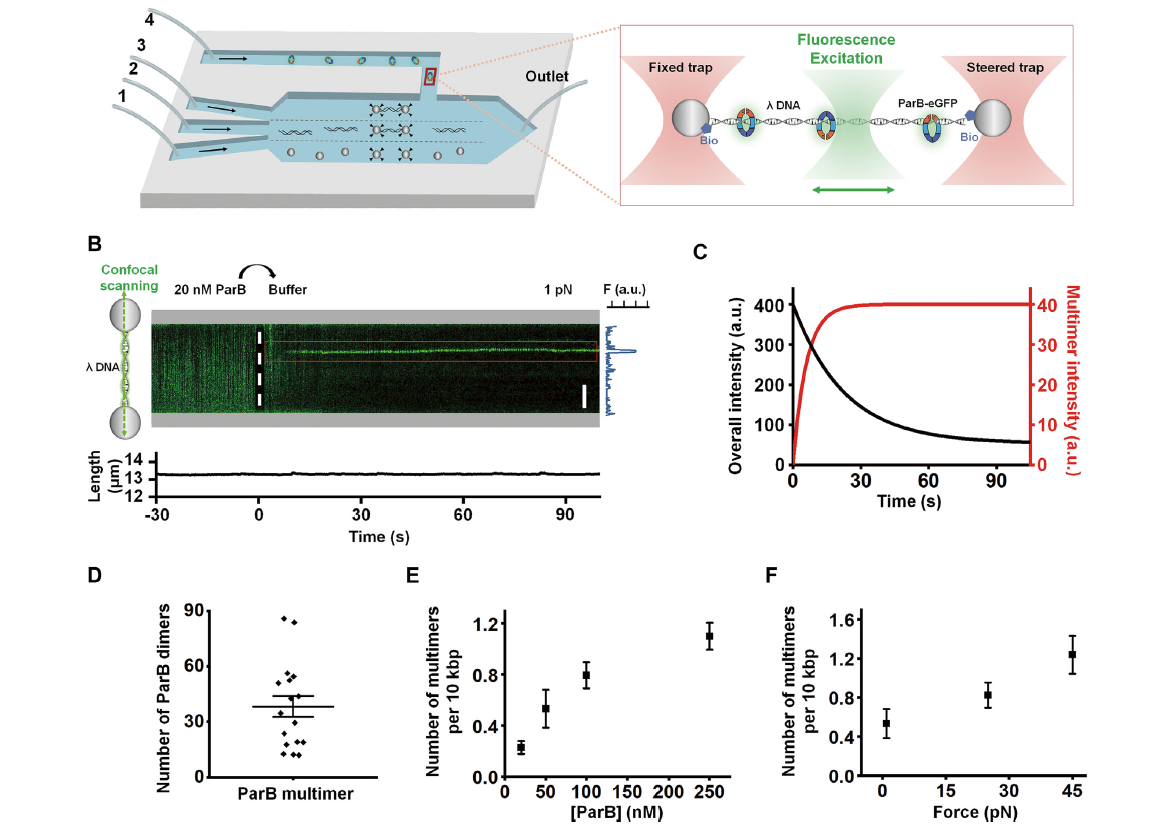
-

-
Heparin induces α-synuclein to form new fibril polymorphs with attenuatedFull Article
neuropathology
Tao Y.Q.¶, Sun Y.P.¶, Lv S.R., Xia W.C., Zhao K., Xu Q.H., Zhao Q.Y., He L., Wang Y.,
Liu C.*, Li D.*
Nat Commun., 2022. (¶co-first author, *corresponding author) 
-

-
Hyperosmotic-stress-induced liquid-liquid phase separation of ALS-relatedFull Article
proteins in the nucleus
Gao C.¶, Gu J.G., Zhang H., Jiang K., Tang L.L., Liu R., Zhang L., Zhang P.F., Liu C.*,
Dai B.*, Song H.*
Cell Reports., 2022. (¶first author, *corresponding author) 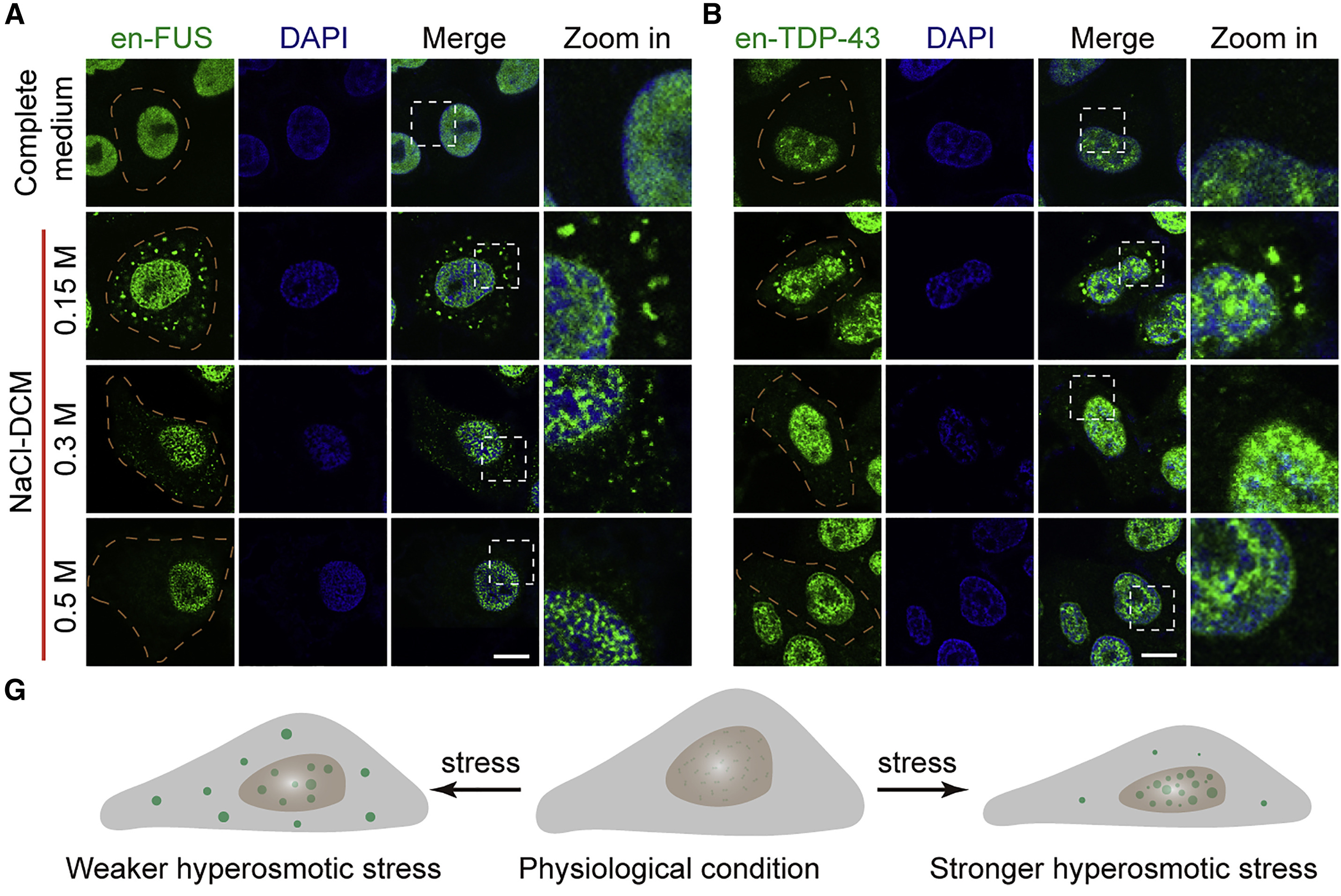
-
-
Structural insights of Fe 3+ induced α-synuclein fibrillation in Parkinson's diseaseFull Article
Zhao Q.Y.¶, Tao Y.Q.¶, Zhao K., Ma Y.Y., Xu Q.H., Liu C., Zhang S.N., Li D.*
J Mol Biol., 2022. (¶co-first author, *corresponding author) 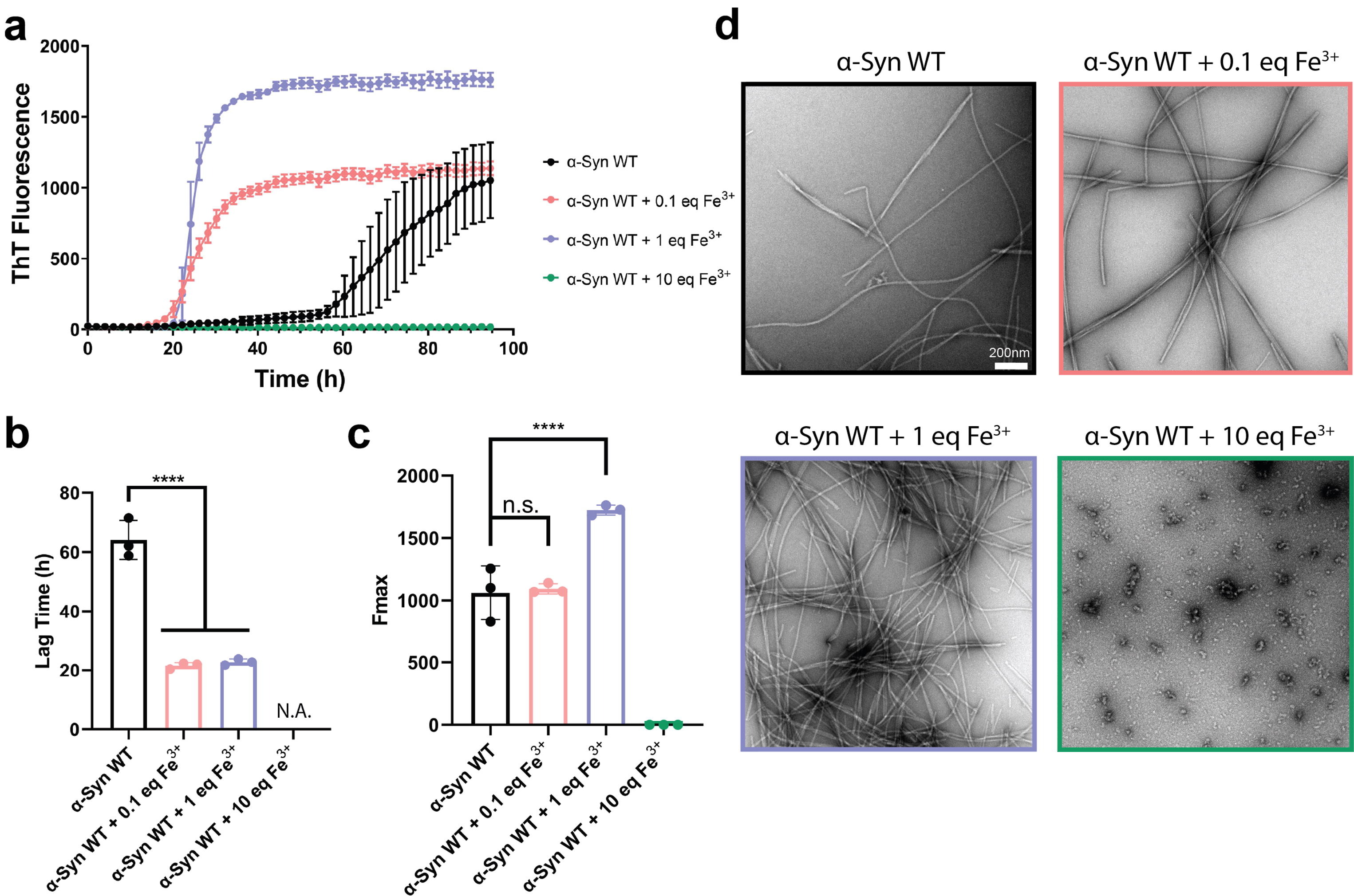
-
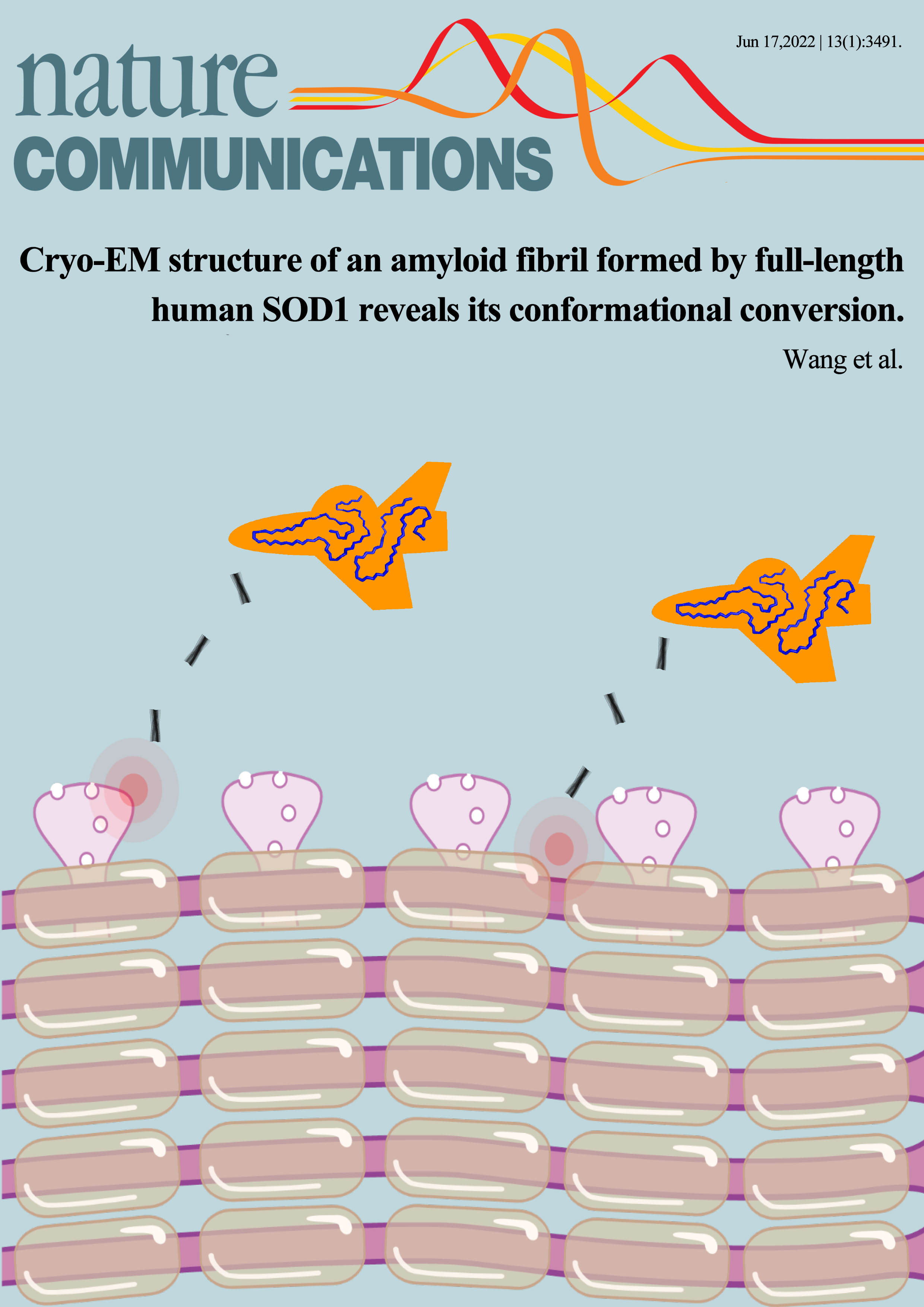
-
Cryo-EM structure of an amyloid fibril formed by full-length human SOD1 reveals its conformational conversionFull Article
Wang L.Q.¶, Ma Y.Y.¶, Yuan H.Y.¶, Zhao K., Zhang M.Y., Wang Q., Huang X., Dai B.,
Chen J., Li D., Zhang D.L., Wang Z.Z., Zou L.Y., Yin P., Liu C.*, Liang Y.*
Nat. Commun., 2022. (¶co-first author, *corresponding author) 
-

-
Hsp70 exhibits a liquid-liquid phase separation ability and chaperones condensed FUS against amyloid aggregationFull Article
Li Y.C.¶, Gu J.G., Wang C., Hu J.J., Zhang S.Q., Liu C., Zhang S.N., Fang Y.S., Li D.*
iScience., 2022. (¶co-first author, *corresponding author) 
-

-
Conformational strains of pathogenic amyloid proteins in neurodegenerativeFull Article
diseases
Li D.*, Liu C.*
Nat Rev Neurosci., 2022. (*corresponding author) 
-

-
Cellular and animal models to investigate pathogenesis of amyloid aggregationFull Article
in neurodegenerative diseases
Long H.F., Zeng S.Y., Li D.*
Biophysics Reports., 2022. (*corresponding author) 
-

-
Biochemical and biophysical characterization of pathological aggregation ofFull Article
amyloid proteins
Long H.F., Zeng S.Y., Sun Y.P., Liu C.*
Biophysics Reports., 2022. (*corresponding author) 
-

-
Identifying Heterozipper β-Sheet in Twisted Amyloid AggregationFull Article
Song Y.X., Dai B., Wang Y., Wang Y., Liu C., Gourdon P., Liu L.*, Wang K.T.*,
Dong M.D.*
Nano Lett., 2022. (*corresponding author) 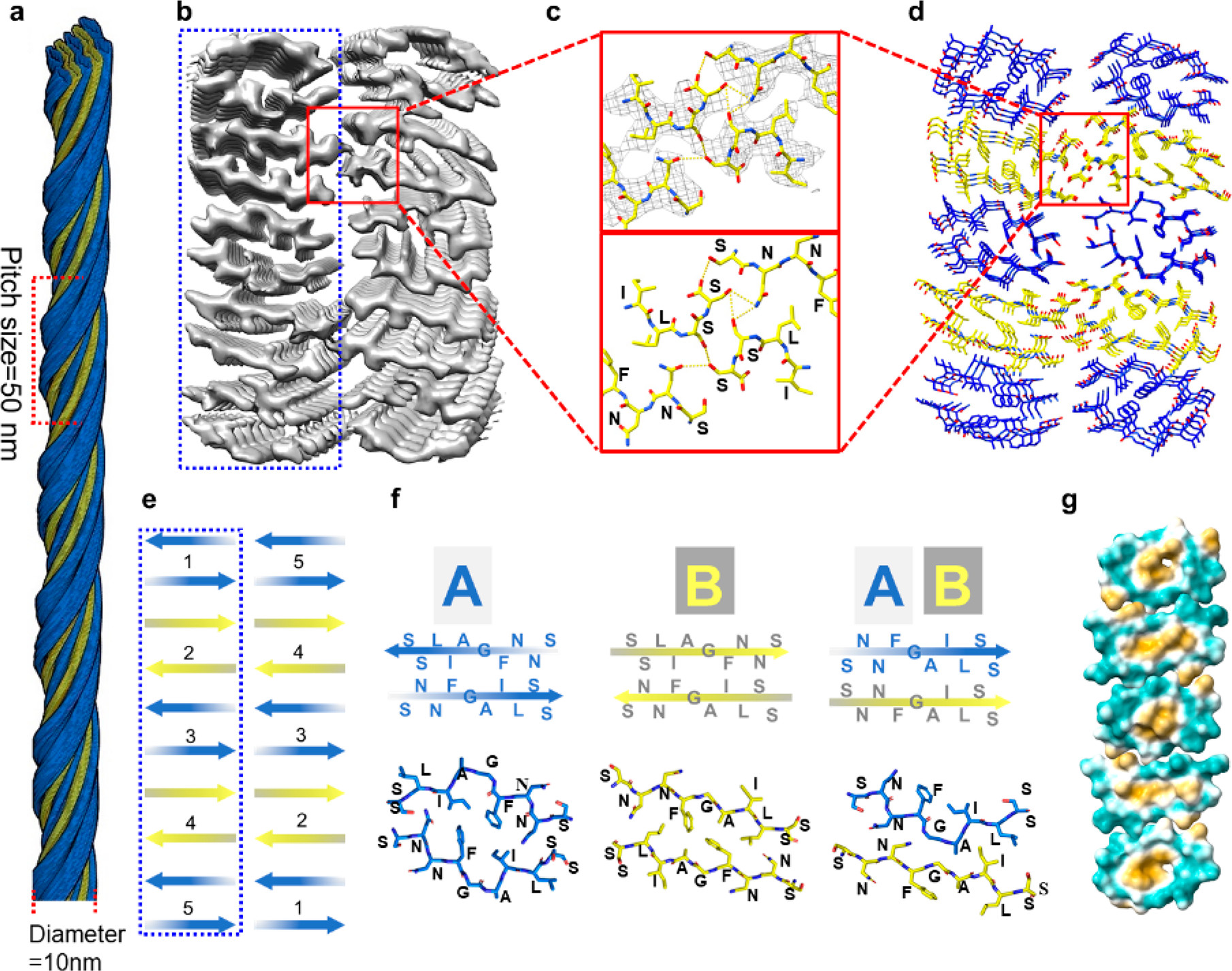
-

-
Liquid-liquid phase separation of RBGD2/4 is required for heat stress resistanceFull Article
in Arabidopsis
Zhu S.B.¶, Gu J.G.¶, Yao J.J., Li Y.C., Zhang Z.T., Xia W.C., Wang Z., Gui X.R., Li L.T.,
Li D., Zhang H.*, Liu C.*
Developmental Cell., 2022. (¶co-first author, *corresponding author) 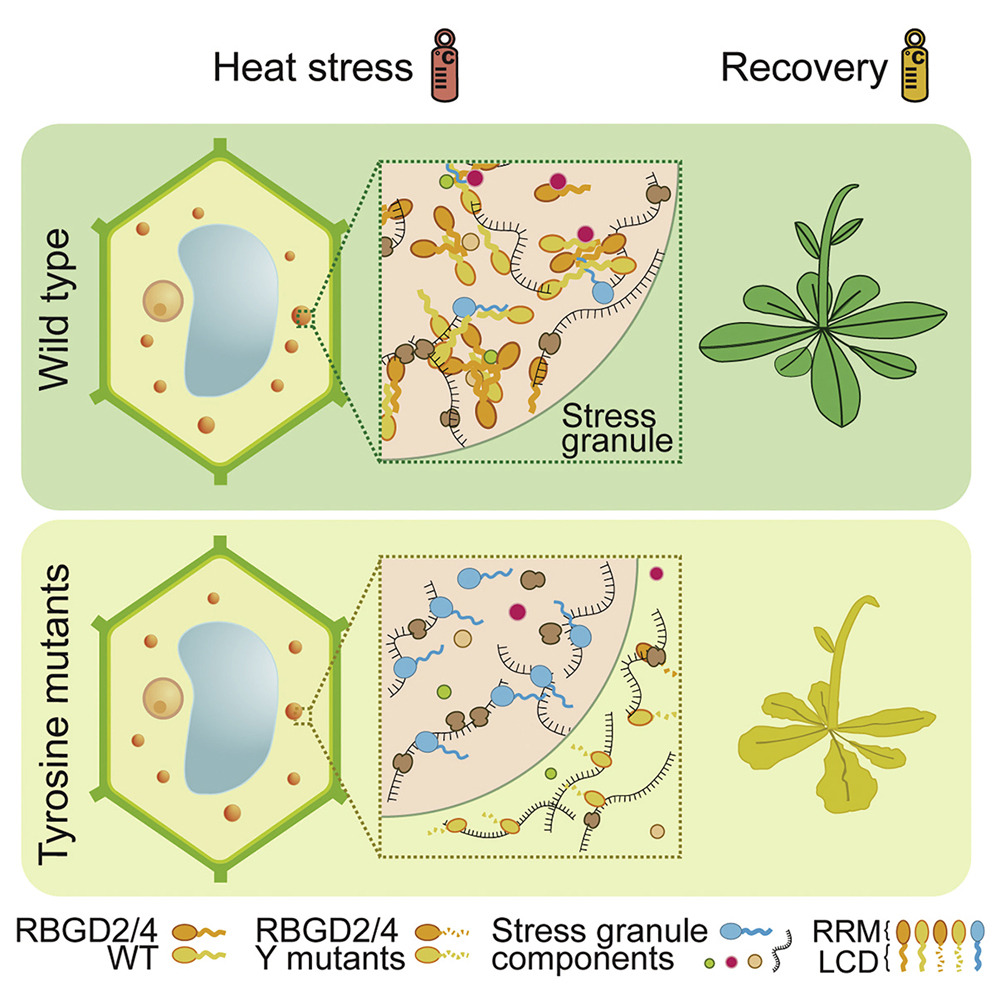
-

-
A high-throughput method for exploring the parameter space of proteinFull Article
liquid-liquid phase separation
Li Y.C.¶, Gu J.G.¶, Liu C.*, Li D.*
Cell Reports Physical Science., 2022 (¶co-first author, *corresponding author) 
-
-
SARS-CoV-2 impairs the disassembly of stress granules and promotesFull Article
ALS-associated amyloid aggregation
Li Y.C.¶, Lu S.Y.¶, Gu J.G.¶, Xia W.C., Zhang S.N., Zhang S.Q., Wang Y., Zhang C.,
Sun Y.P., Lei J., Liu C., Su Z.M.*, Yang J.T.*, Peng X.Z.*, Li D.*
Protein Cell., 2022. (¶co-first author, *corresponding author) 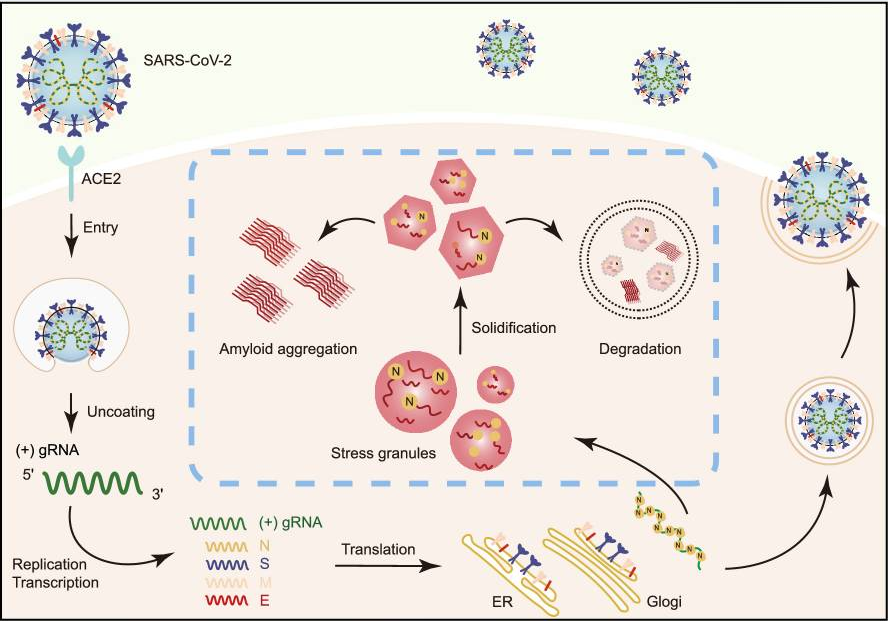
-

-
The mouse nicotinamide mononucleotide adenylyltransferase chaperonesFull Article
diverse pathological amyloid client proteins
Huang C.A.¶, Lu J.X.¶, Ma X.J.¶, Qiang J.L., Wang C.C., Liu C., Fang Y.S., Zhang Y.Y.,
Li D.*, Zhang S.N.*
J Biol Chem., 2022. (¶co-first author, *corresponding author) 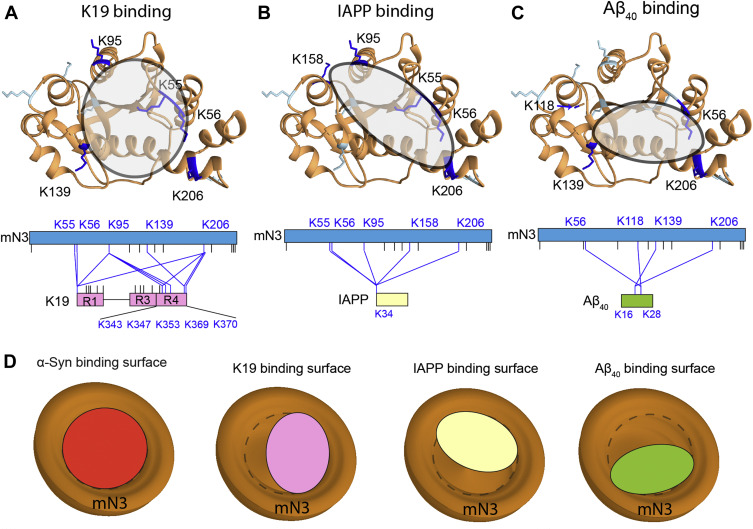
-
-
Generic amyloid fibrillation of TMEM106B in patient with Parkinson's diseaseFull Article
dementia and normal elders
Fan Y.¶, Zhao Q.Y.¶, Xia W.C.¶, Tao Y.Q., Yu W.B., Chen M.J., Liu Y.Q., Zhao J.,
Sun Y.P., Si C.F., Zhang S.Q., Zhang Y.Y., Li W.S., Liu C.*, Wang J.*, Li D.*
Cell Research., 2022. (¶co-first author, *corresponding author) 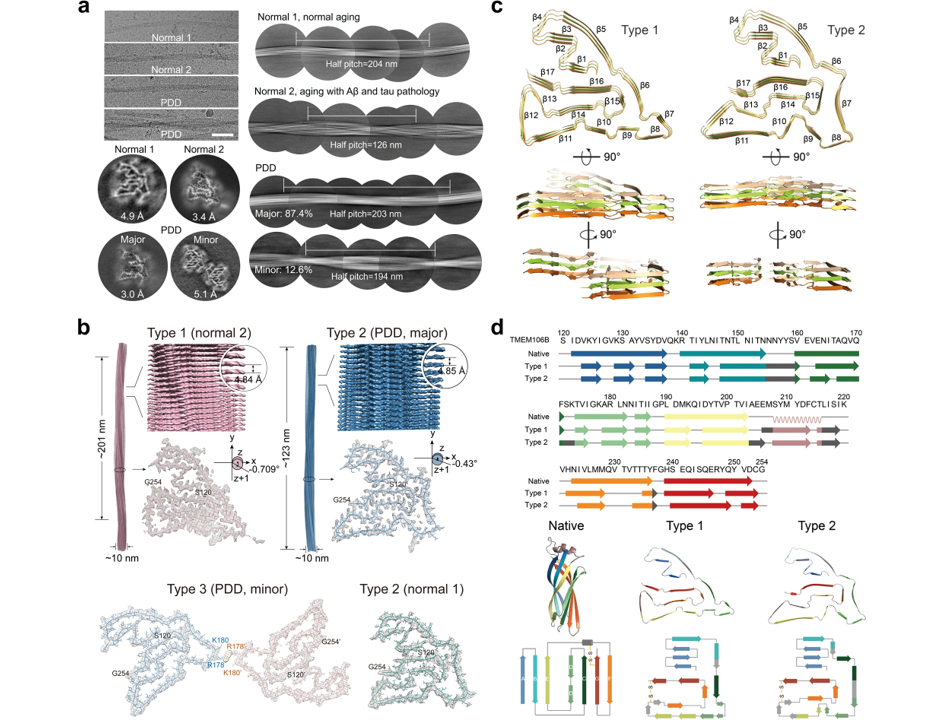
-

-
Spatiotemporal dynamic regulation of membraneless organelles by chaperoneFull Article
networks
Li D.*, Liu C.*
Trends in Cell Biology., 2022. (*corresponding author) 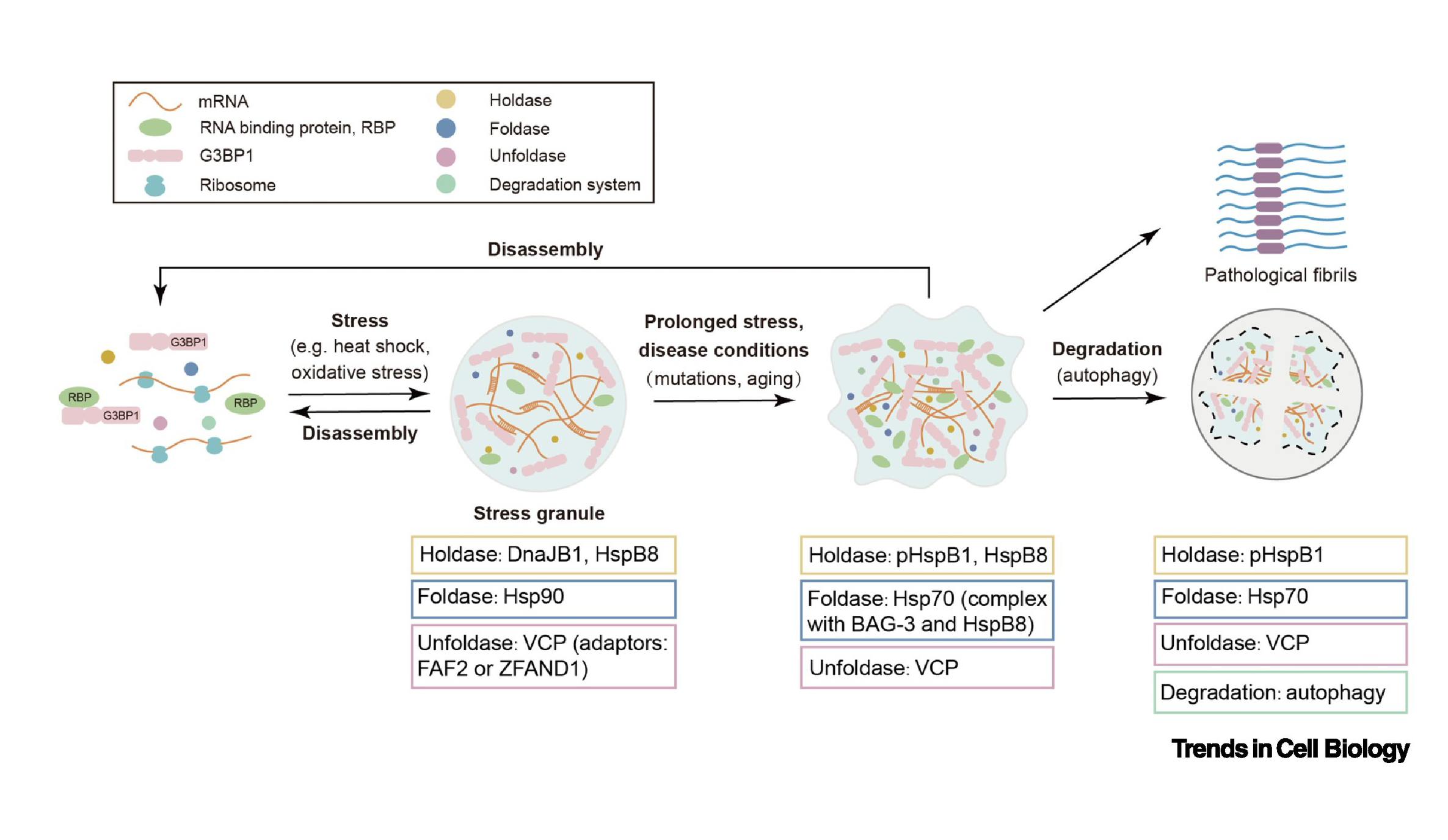
-

-
Molecular structure of an amyloid fibril formed by FUS low-complexity domainFull Article
Sun Y.P.¶, Zhang S.Q.¶, Hu J.J.¶, Tao Y.Q., Xia W.C., Gu J.G., Li Y.C., Cao Q., Li D.,
Liu C.*
iScience., 2021. (¶co-first author, *corresponding author) 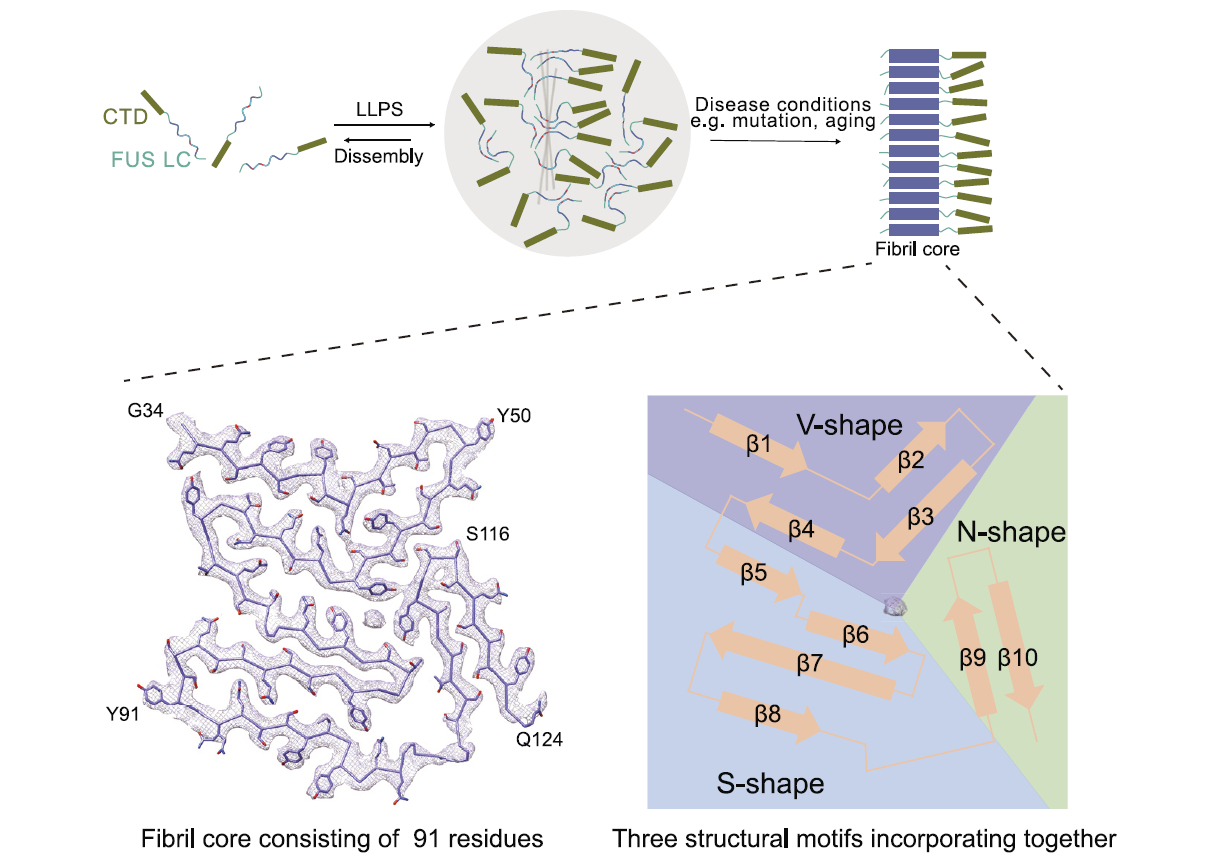
-
-
Genetic prion disease–related mutation E196K displays a novel amyloid fibrilFull Article
structure revealed by cryo-EM
Wang L.Q.¶, Zhao K.¶, Yuan H.Y.¶, Dang H.B., Ma Y.Y., Wang Q., Wang C., Sun Y.P., Chen J., Li D., Zhang D.L., Yin P., Liu C.*, Liang Y.*
Science Advances., 2021. (¶co-first author, *corresponding author) 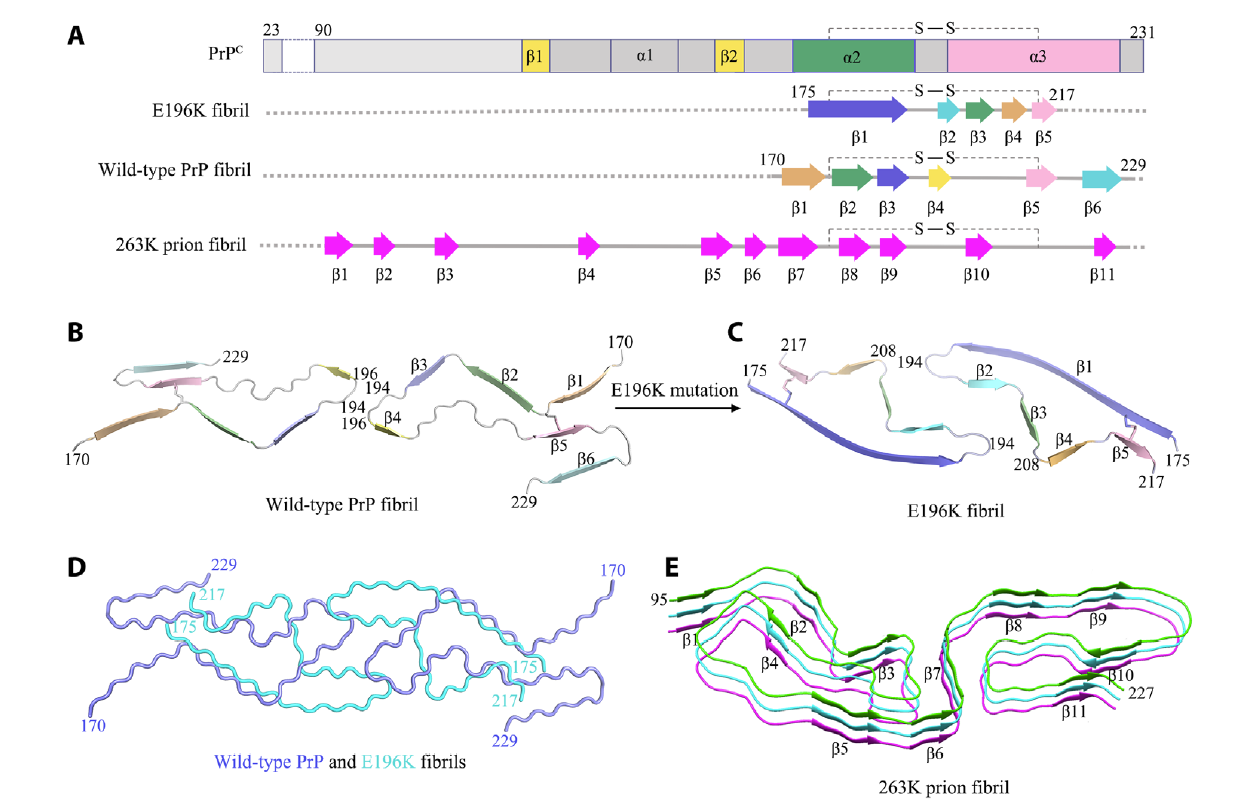
-
-
The hereditary mutation G51D unlocks a distinct fibril strain transmissible toFull Article
wild-type α-synuclein
Sun Y.P.¶, Long H.F.¶, Xia W.C., Wang K., Zhang X., Sun B., Cao, Q., Zhang Y.Y.,
Dai B., Li D., Liu C.*
Nat. Commun., 2021. (¶co-first author, *corresponding author) 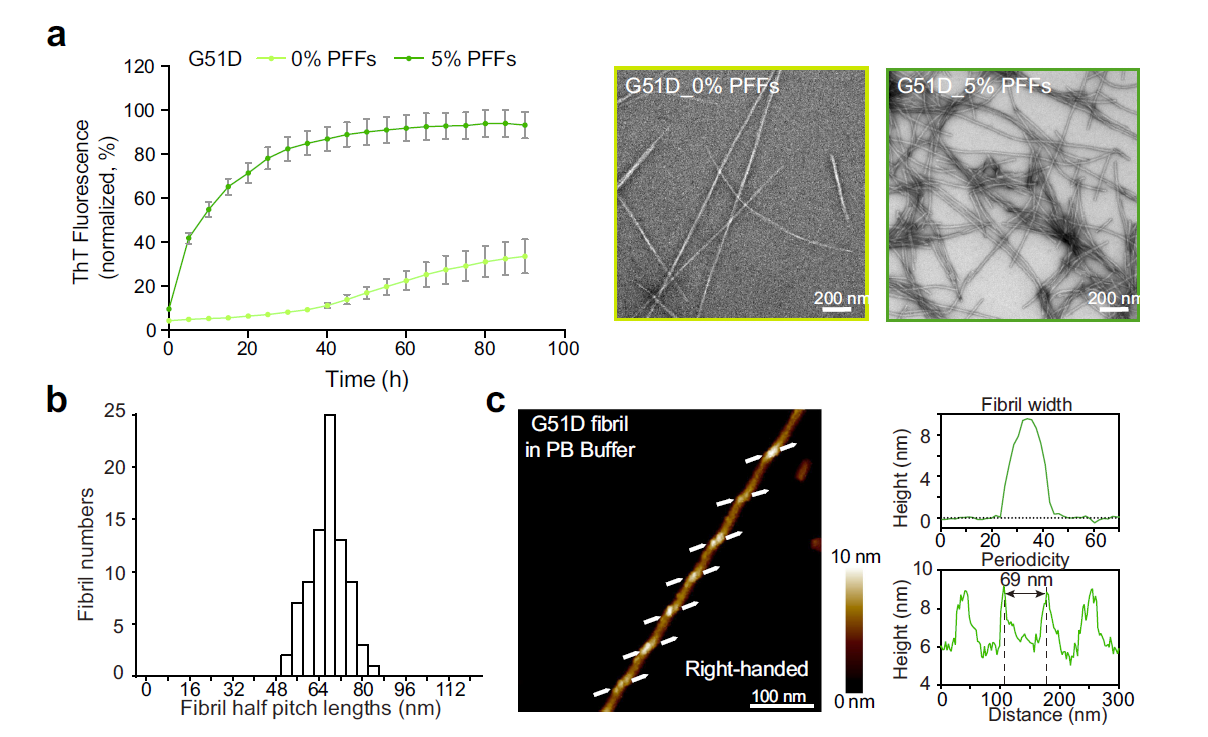
-
-
O-Glycosylation Induces Amyloid-β To Form New Fibril Polymorphs VulnerableFull Article
for Degradation
Liu D.L.¶, Wei Q.J.¶, Xia W.C.¶, He C.D., Zhang Q.K., Huang L., Wang X.Y., Sun Y.P.,
Ma Y.Y., Zhang X.H., Wang Y., Shi X.M., Liu C.*, Dong S.W.*
J. Am. Chem. Soc., 2021. (¶co-first author, *corresponding author) 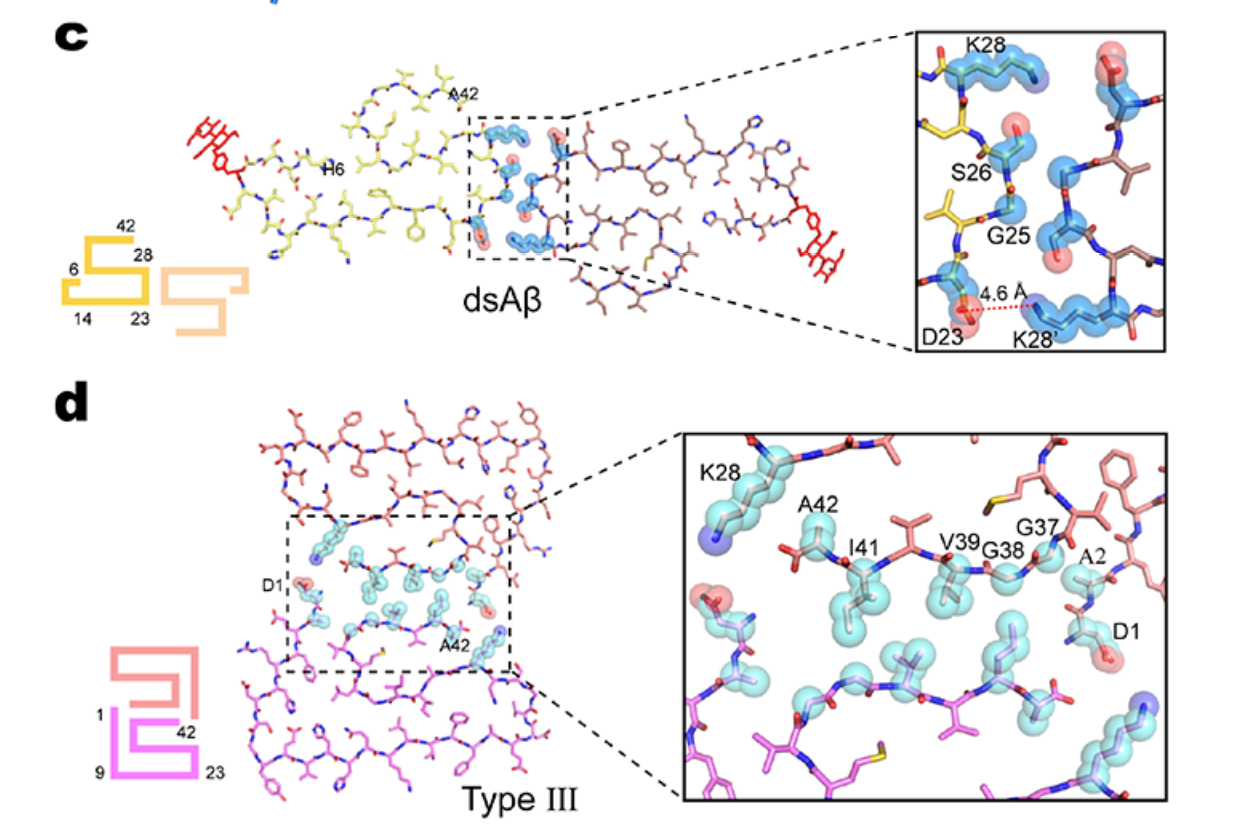
-
-
Hsp70 chaperones TDP-43 in dynamic, liquid-like phase and prevents it fromFull Article
amyloid aggregation
Gu J.G.¶, Wang C.¶, Hu R.F.¶, Li Y.C., Zhang S.N., Sun Y.P., Wang Q.Q., Li D.,
Fang Y.S.*, Liu C.*
Cell Research., 2021. (¶co-first author, *corresponding author) 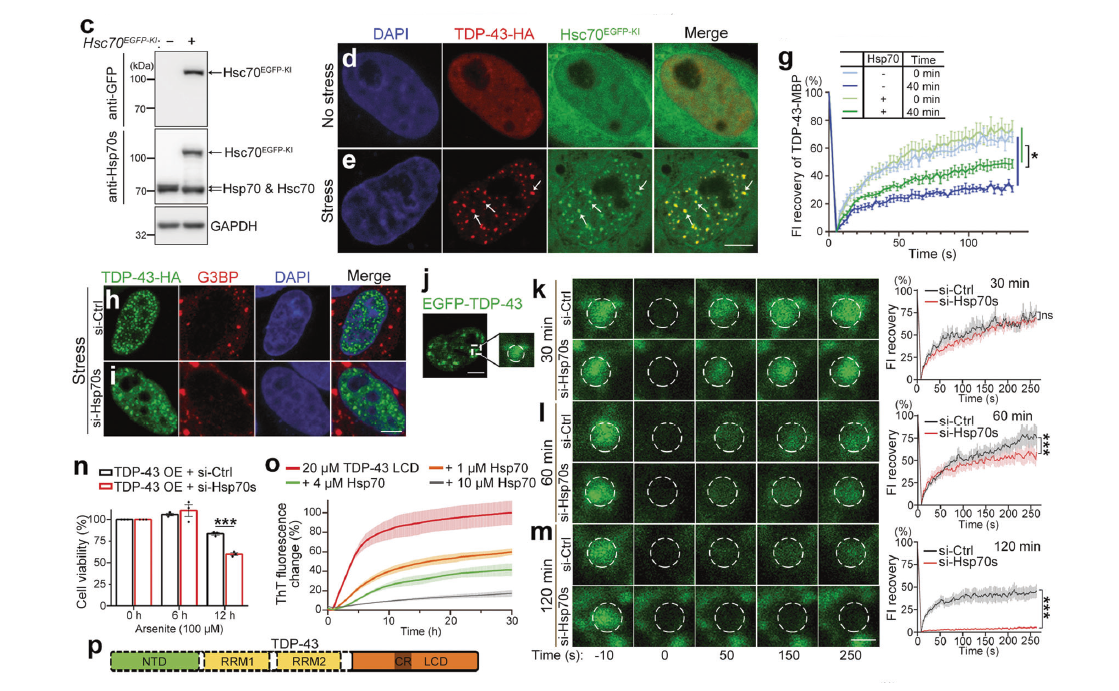
-

-
Mechanistic basis for receptor-mediated pathological α-synuclein fibrilFull Article
cell-to-cell transmission in Parkinson's disease
Zhang S.N.¶, Liu Y.Q.¶, Jia C.Y.¶, Lim Y.J.¶, Feng G.Q.¶, Xu E.Q., Long H.F.,
Yasuyoshi Kimura, Tao Y.Q., Zhao C.Y., Wang C.C., Liu Z.Y., Hu J.J., Ma M.R.,
Liu Z.J., Lin J., Li D., Wang R.X., Valina L Dawson, Ted M Dawson*, Li Y.M.*,
Mao X.B.*, Liu C.*
Proc. Natl. Acad. Sci. U S A., 2021. (¶co-first author, *corresponding author) 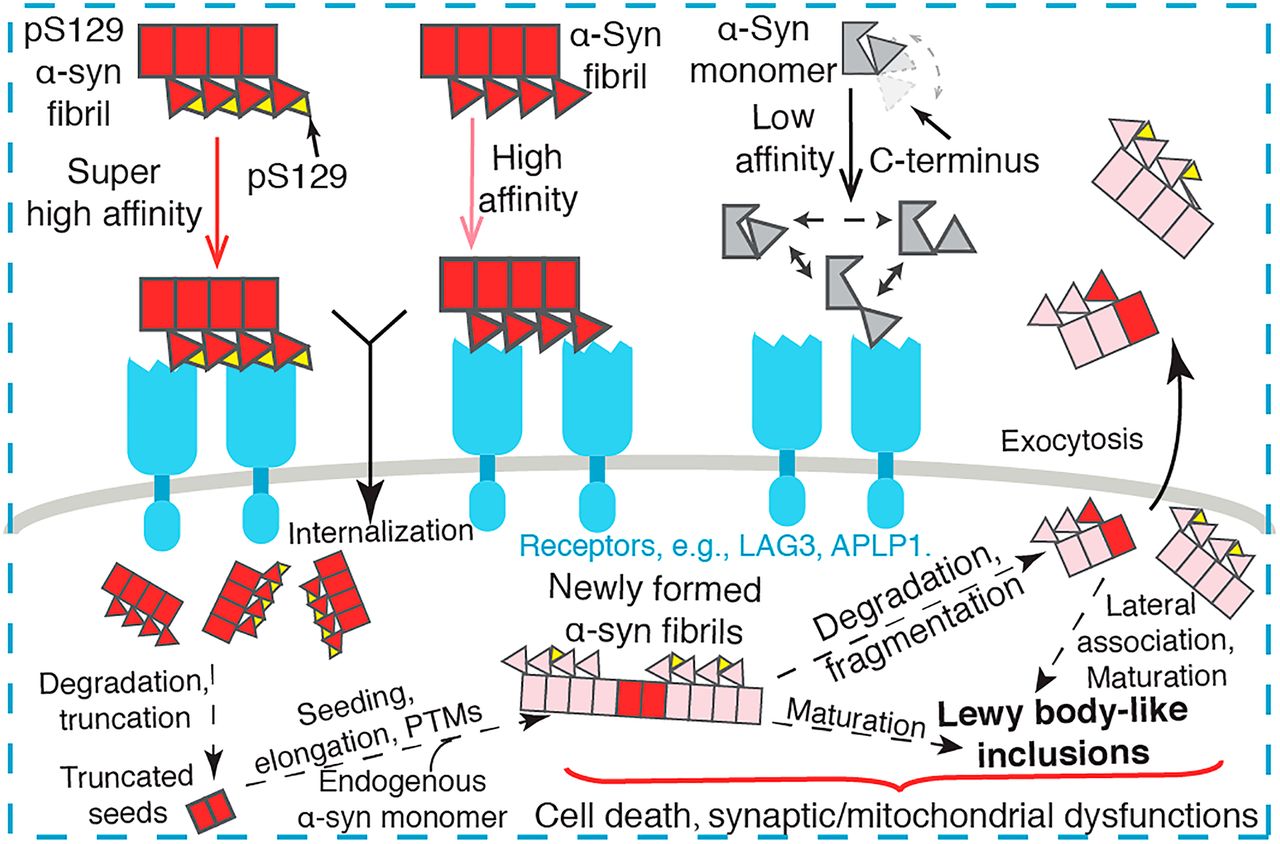
-

-
Wild-type α-synuclein inherits the structure and exacerbated neuropathology of E46K mutant fibril strain by cross-seedingFull Article
Long H.F.¶, Zheng W.T.¶, Liu Y., Sun Y.P., Zhao K., Liu Z.Y., Xia W.C., Lv S.R., Liu Z.T., Li D., He K.W.*, Liu C.*
Proc. Natl. Acad. Sci. U S A., 2021. (¶co-first author, *corresponding author) 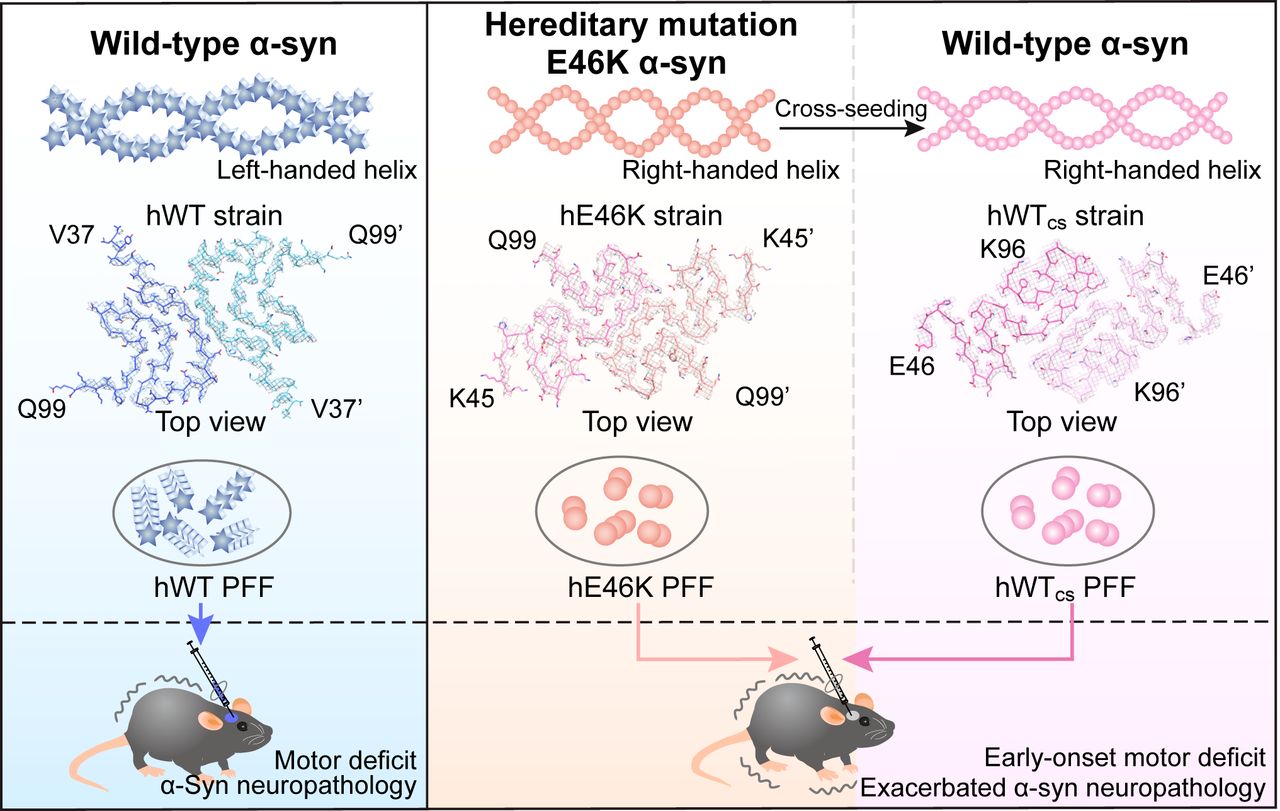
-
-
The structure of a minimum amyloid fibril core formed by necroptosis-mediating RHIM of human RIPK3Full Article
Wu X.L.¶, Ma Y.Y.¶, Zhao K.¶, Zhang J., Sun Y.P., Li Y.C., Dong X.Q., Hu H., Liu J.,
Wang J., Zhang X., Li B., Wang H.Y., Li D., Sun B., Lu J.X.*, Liu C.*
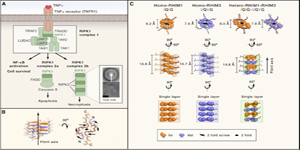
Proc. Natl. Acad. Sci. U S A., 2021. (¶co-first author, *corresponding author) 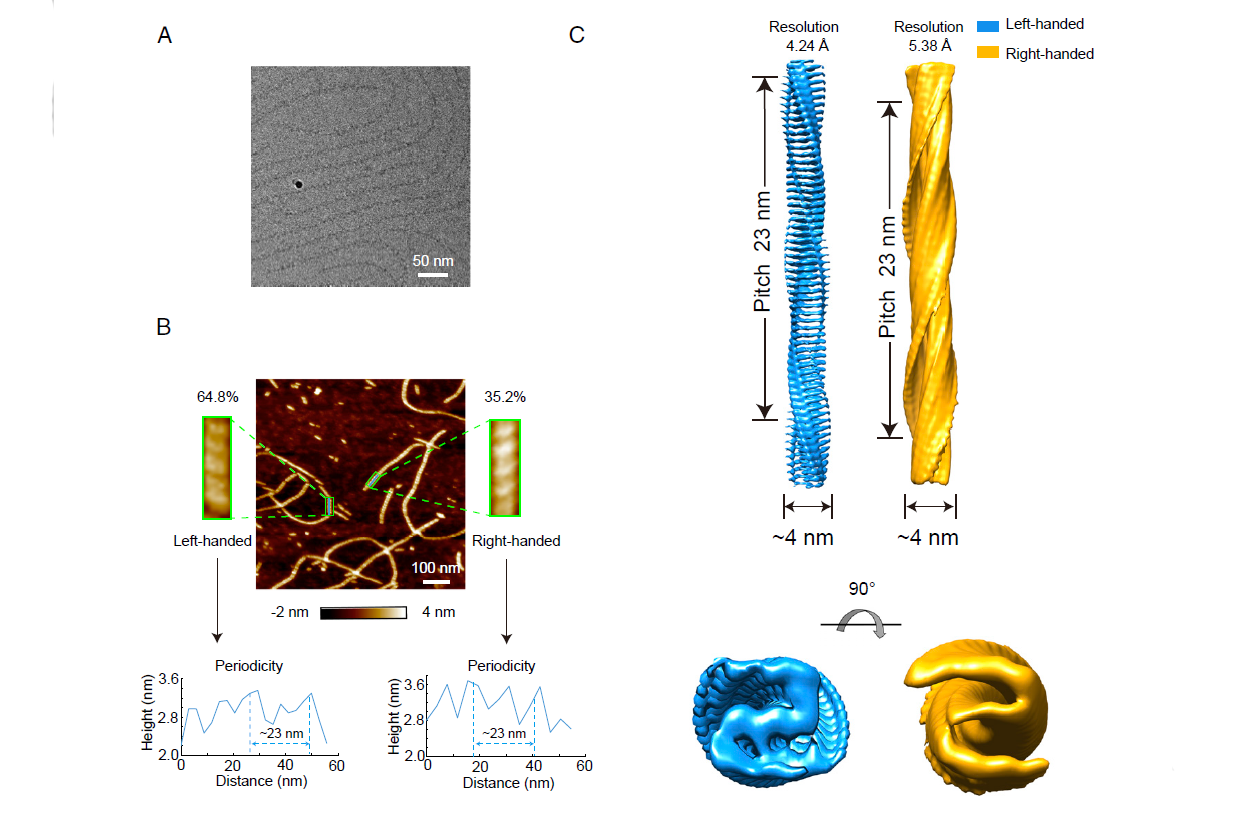
-

-
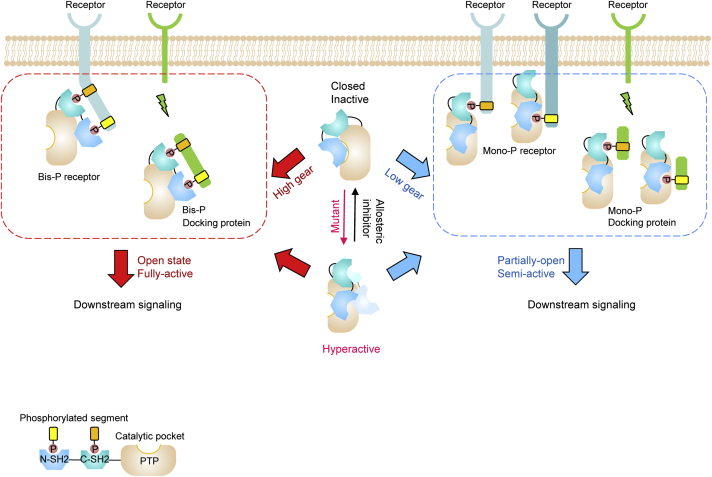
Full Article
A novel partially-open state of SHP2 points to a "multiple gear" regulation
mechanism
Tao Y.Q.¶, Xie J.F.¶, Zhong Q.L.¶, Wang Y.Y.¶, Zhang S.N, Luo F., Wen F.C., Xie J.J.,
Zhao J.W., Sun X.O., Long H.F., Ma J.F., Zhang Q., Long J.G., Fang X.Y., Lu Y., Li D., Li M., Zhu J.D., Sun B., Li G.H.*, Diao J.J.*, Liu C.*
J. Biol. Chem., 2021. (¶co-first author, *corresponding author)
-

-
Full Article
Hierarchical chemical determination of amyloid polymorphs in
neurodegenerative disease
Li D.*, Liu C.*
Nat. Chem. Biol., 2021. (*corresponding author) 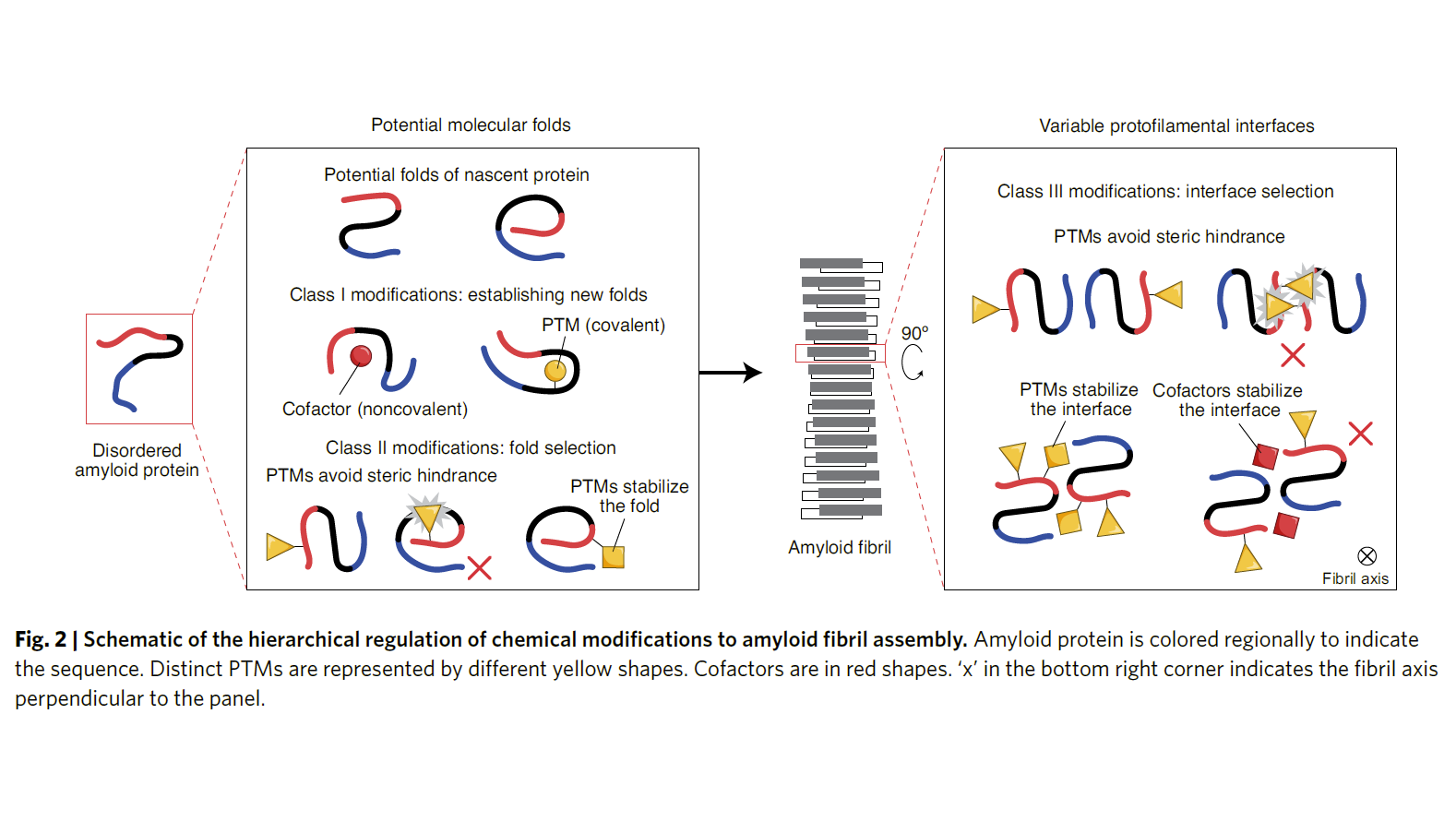
-

-
Full Article
The nuclear localization sequence mediates hnRNPA1 amyloid fibril formation
revealed by cryoEM structure
Sun Y.P.¶, Zhao K.¶, Xia W.C., Feng G.Q., Gu J.G., Ma Y.Y., Gui X.R., Zhang X.,
Fang Y.S., Sun B., Wang R.X., Liu C.*, Li D.*
Nat. Commun., 2020. (¶co-first author, *corresponding author) 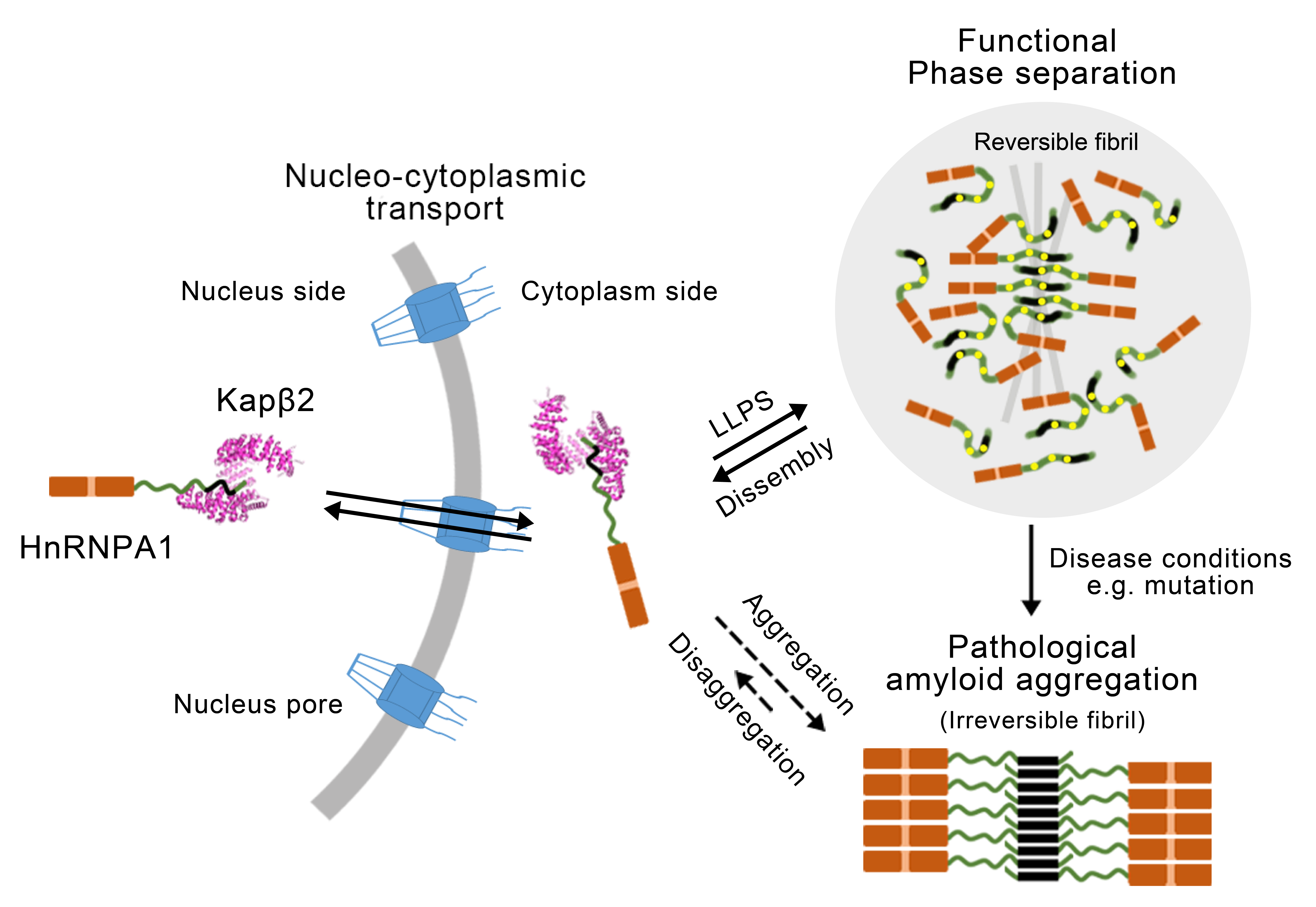
-
-
Full Article
Hsp40 proteins phase separate to chaperone the assembly and maintenance of
membraneless organelles
Gu J.G.¶, Liu Z,Y.¶, Zhang S.N., Li Y.C., Xia W.C., Wang C., Xiang H.J., Liu Z.J., Tan L., Fang Y.S., Liu C.*, Li D.*
Proc. Natl. Acad. Sci. USA., 2020, 117(49), 31123-31133.
(¶co-first author, *corresponding author) 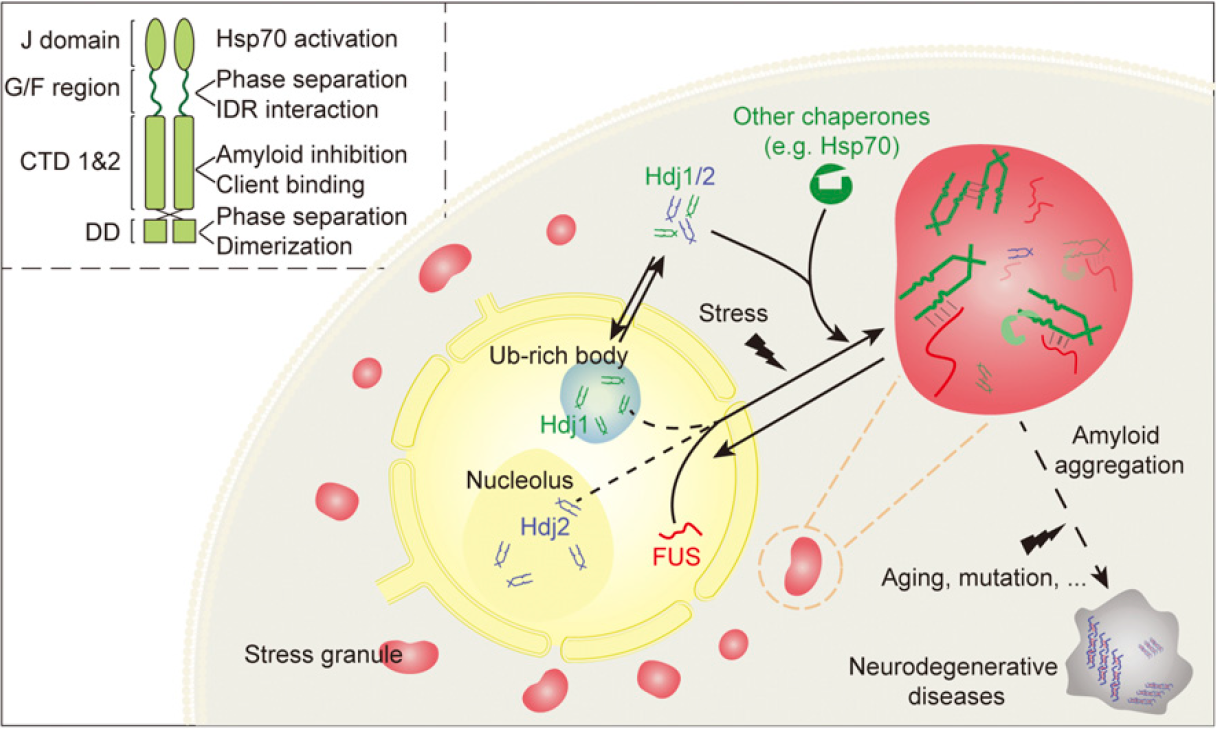
-

-
Full Article
Phase separation of disease-associated SHP2 mutants underlies
MAPK hyperactivation
Zhu G.Y.¶, Xie J.J.¶, Kong W.N.¶, Xie J.F.¶, Li Y.C., Du L., Zheng Q.G., Sun L.,
Guan M.F., Li H., Zhu T.X., He H., Liu Z.Y., Xia X., Kan C., Tao Y.Q.,Shen H.C., Li D.,
Wang S.Y., Yu Y.G., Yu Z.H., Zhang Z.Y., Liu C.*, Zhu J.D.*
Cell,2020. (¶co-first author, *corresponding author) 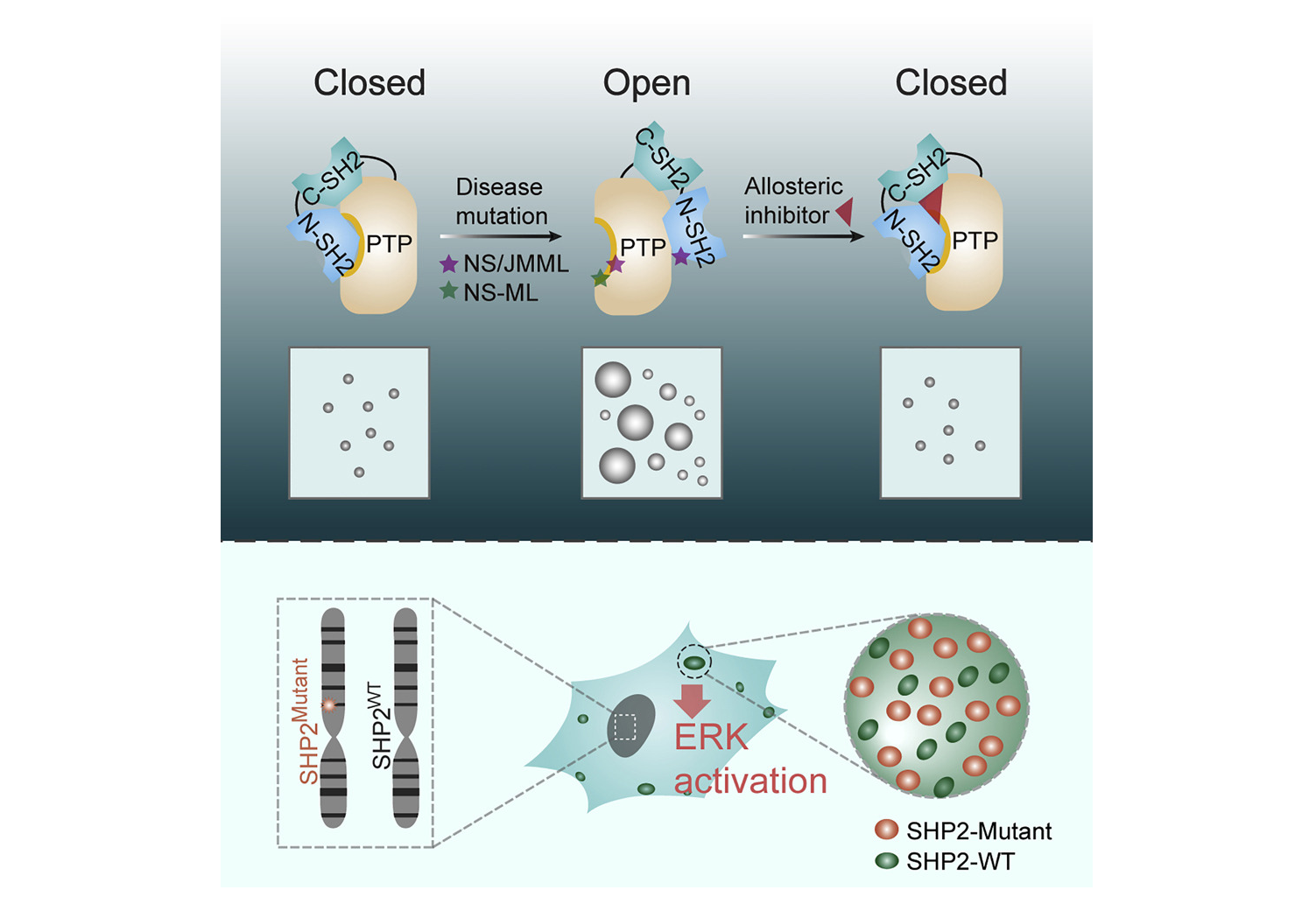
-
-
Parkinson's disease-related phosphorylation at Tyr39 rearranges α-synucleinFull Article
amyloid fibril structure revealed by cryo-EM
Zhao K.¶, Lim Y.J.¶, Liu Z.Y.¶, Long H.F., Sun Y.P., Hu J.J., Zhao C.Y., Tao Y.Q.,
Zhang X., Li D., Li Y.M.*, Liu C.*
Proc. Natl. Acad. Sci. USA.,2020, 117(33), 20305-20315.
(¶co-first author, *corresponding author) 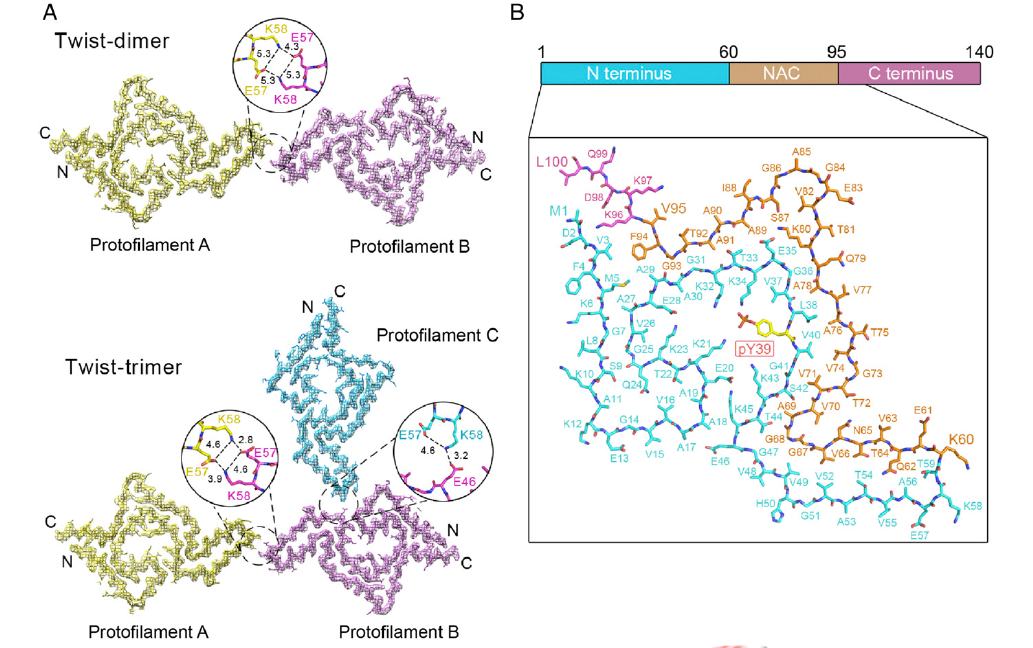
-

-
Liquid-liquid Phase Separation in Biology: Mechanisms, Physiological FunctionsFull Article
and Human Diseases
Zhang H.*, Ji X.*, Li P.*, Liu C.*, Lou J.*, Wang Z., Wen W.*, Xiao Y.,
Zhang M.*, Zhu X.*
Sci. China. Life. Sci., 2020, 63, 953–985.(*corresponding author) 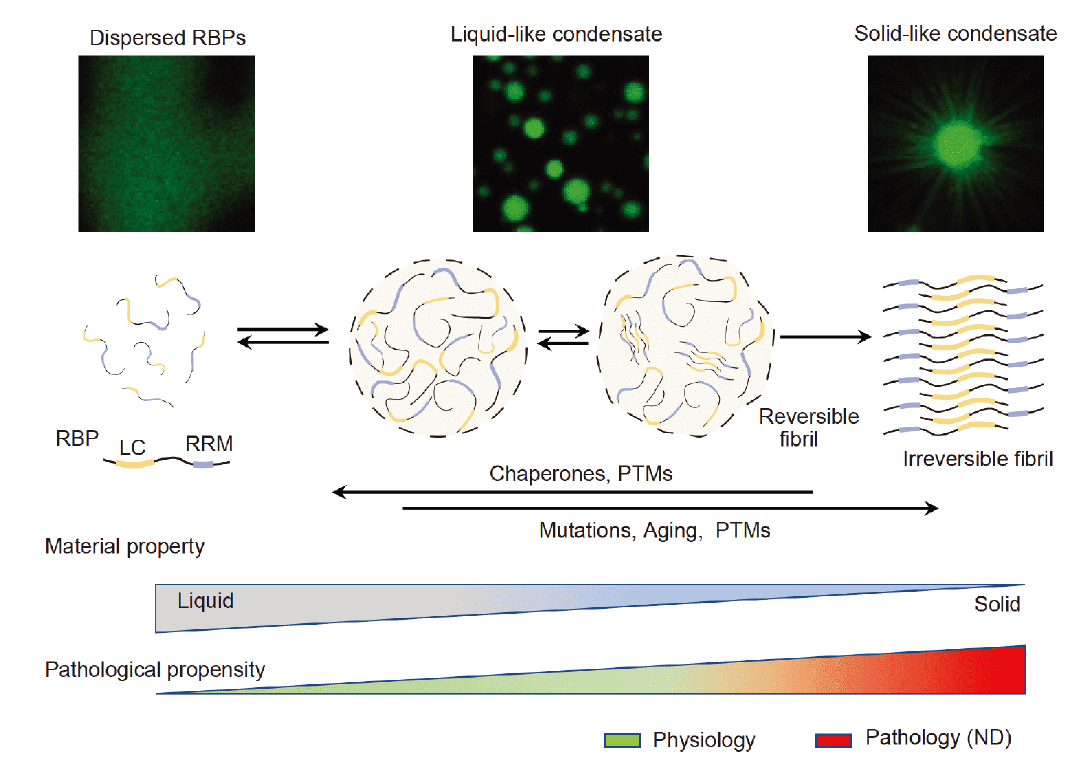
-
-
Stress Induces Dynamic, Cytotoxicity-Antagonizing TDP-43 Nuclear Bodies viaFull Article
Paraspeckle LncRNA NEAT1-Mediated Liquid-Liquid Phase Separation
Wang C.¶, Duan Y.J.¶, Duan G.¶, Wang Q.Q., Zhang K., Deng X., QianB.T., Gu J.G., Ma Z.W., Zhang S., Guo L., Liu C.*, Fang Y.S.*
Mol. Cell., 2020.(¶co-first author, *corresponding author) 
-
-
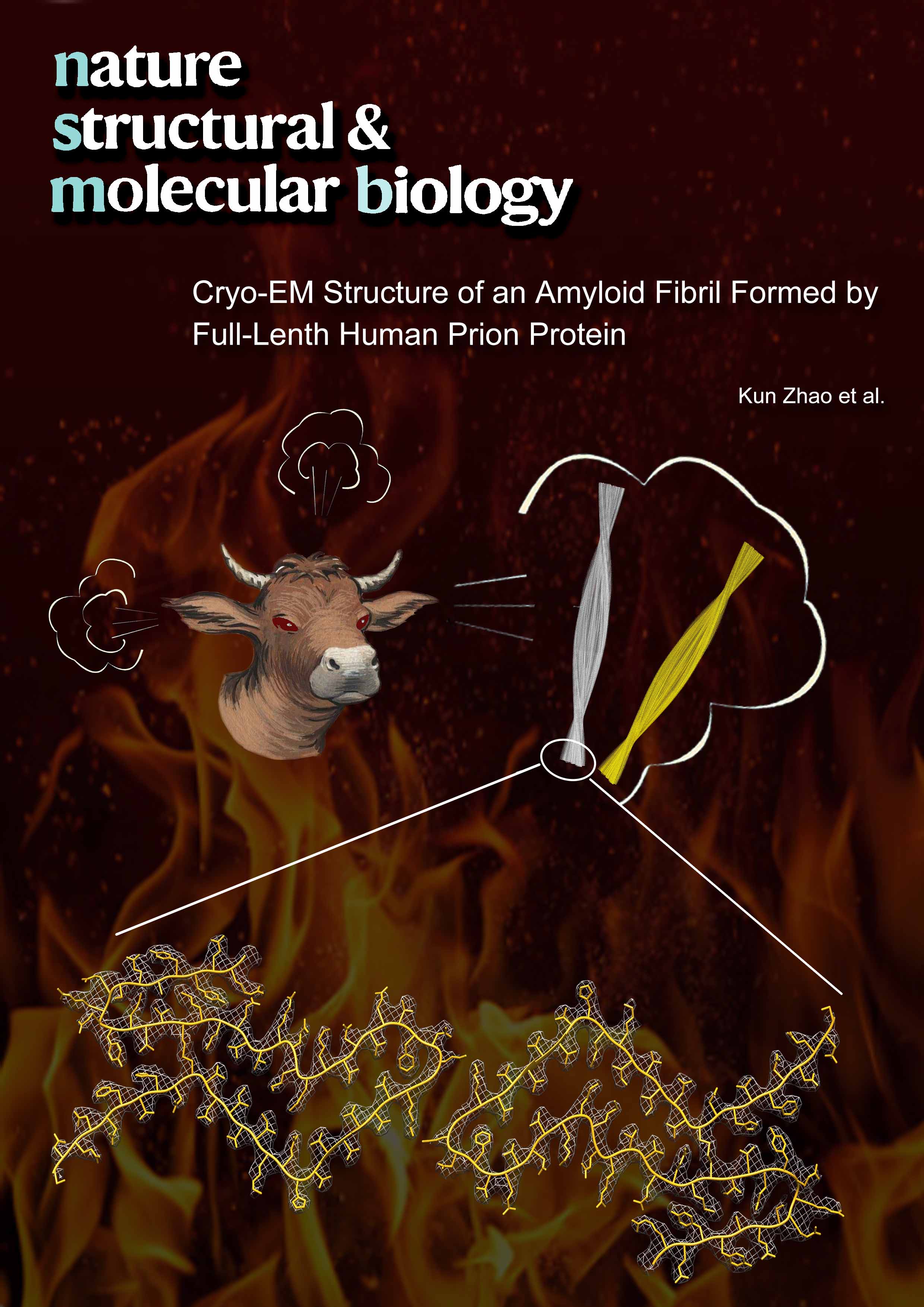
Cryo-EM Structure of an Amyloid Fibril Formed by Full-LengthFull Article
Human Prion Protein
Wang L.Q.¶, Zhao K.¶, Yuan H.Y., Wang Q., Guan Z., Tao J., Li X.N.,
Sun Y.P., Yi C.W., Chen J., Li D., Zhang D., Yin P., Liu C.*, Liang Y.*
Nat. Struct. Mol. Biol., 2020, 27, 598–602.
(¶co-first author, *corresponding author) 
-

-
Parkinson’s disease associated mutation E46K of α-synuclein triggersFull Article
the formation of a distinct fibril structure
Zhao K.¶, Li Y.W.¶, Liu Z.Y., Long H.F., Zhao C.Y., Luo F., Sun Y.P., Tao Y.Q., Su X.D., Li D.*, Li X.M.*, Liu C.*
Nat. Commun., 2020, 11, 2643. (¶co-first author,
*corresponding author) 
-

-
Structural basis of the interplay between α-synuclein and Tau inFull Article
regulating pathological amyloid aggregation
Lu J.X.¶, Zhang S.N.¶, Ma X.J.¶, Jia C.Y., Liu Z.Y., Huang C.A., Liu C.*, Li D.*
J. Biol. Chem., 2020, bc.RA119.012284. (¶co-first author,
*corresponding author) 
-

-
Full Article
Nicotinamide mononucleotide adenylyltransferase uses its
NAD+ substrate-binding site to chaperone phosphorylated Tau
Ma X.J.¶, Zhu Y.¶, Lu J.X., J.F., Li C., Shin W.S., Qiang J.L, Liu J.Q., Dou S.,
Xiao Y., Wang C.C., Jia C.Y., Long H.F., Yang J.T., Fang Y.S., Jiang L.,
Zhang Y.Y., Zhang S.N., Zhai R.G.*, Liu C.*, Li D.*
eLife., 2020;9:e51859 (¶co-first author, *corresponding author)
-

-
Hsp27 chaperones FUS phase separation under the modulation ofFull Article
stress-induced phosphorylation
Liu Z.Y.¶, Zhang S.N.¶, Gu J.G.¶, Tong Y.L., Li Y.C., Gui X.R., Long H.F.,
Wang C.C., Zhao C.Y., Lu J.X., He L., Li Y., Liu Z.J., Li D.*, Liu C.*Nat. Struct. Mol. Biol., 2020, 27, 363–372.(¶co-first
author, *corresponding author)

-
-
Different regions of synaptic vesicle membrane regulate VAMP2Full Article
conformation for the SNARE assembly
Wang C.C.¶, Tu J., Zhang S.N., Cai B., Liu Z.Y., Hou S.Q., Zhong Q.L.,
Hu X., Liu W.B., Li G.H., Liu Z.J., He L., Diao J.J., Zhu Z.J., Li D.*, Liu C.*
Nat. Commun., 2020, 11, 1531.(¶first author, *corresponding author) 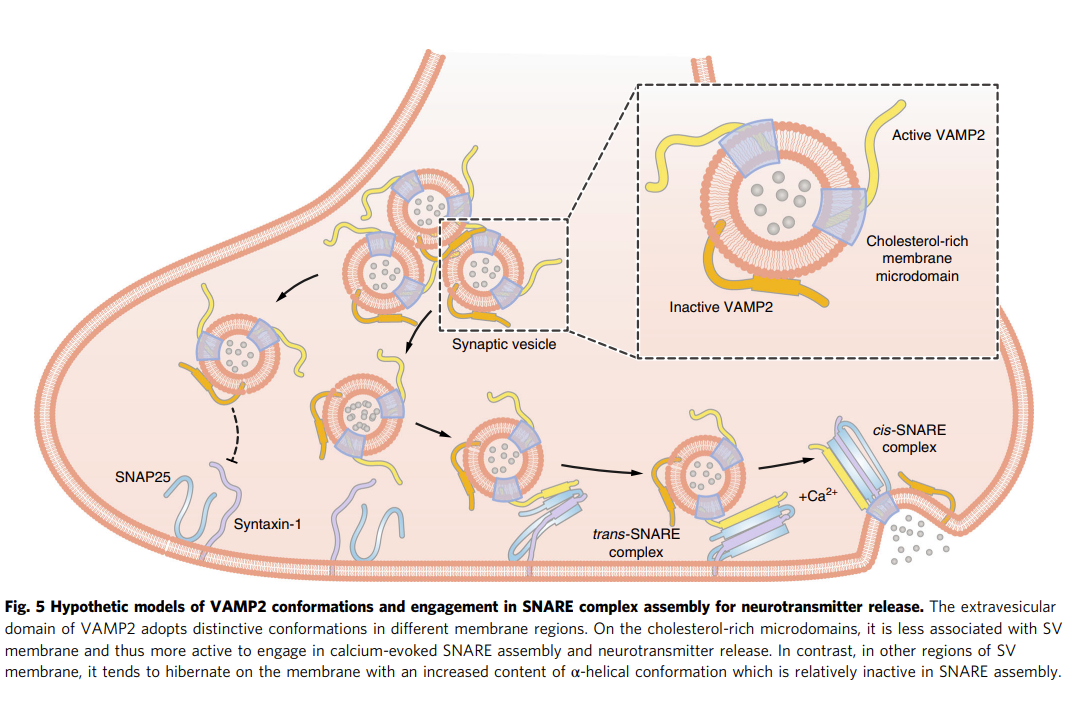
-

-
Cryo-EM structure of full-length α-synuclein amyloid fibril withFull Article
Parkinson's disease familial A53T mutation
Sun Y.P.¶, Hou S.Q.¶, Zhao K.¶, Long H.F., Liu Z.Y., Gao J., Zhang Y.Y.,
Su X.D., Li D.*, Liu C.*Cell Research, 2020, 30, 360–362.(¶co-first author, *corresponding
author)
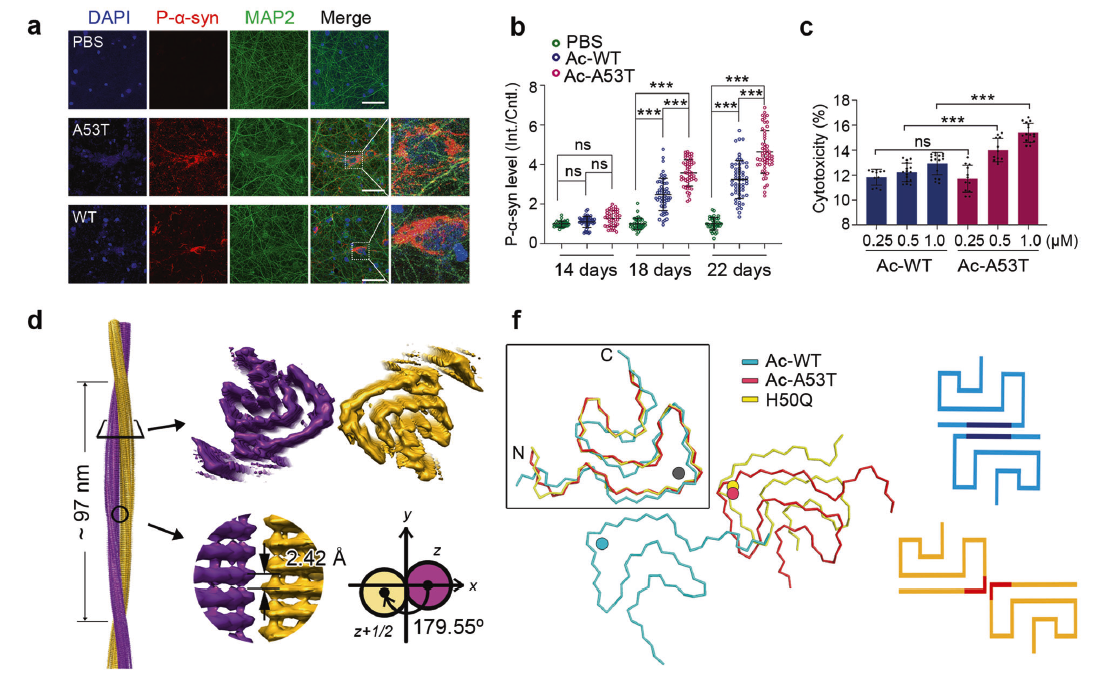
-

-
Structural diversity of amyloid fibrils and advances in their structureFull Article
determination (Invited Perspective)
Li D.*, Liu C.*
Biochemistry, 2020, 59(5), 639-646.(*corresponding author) 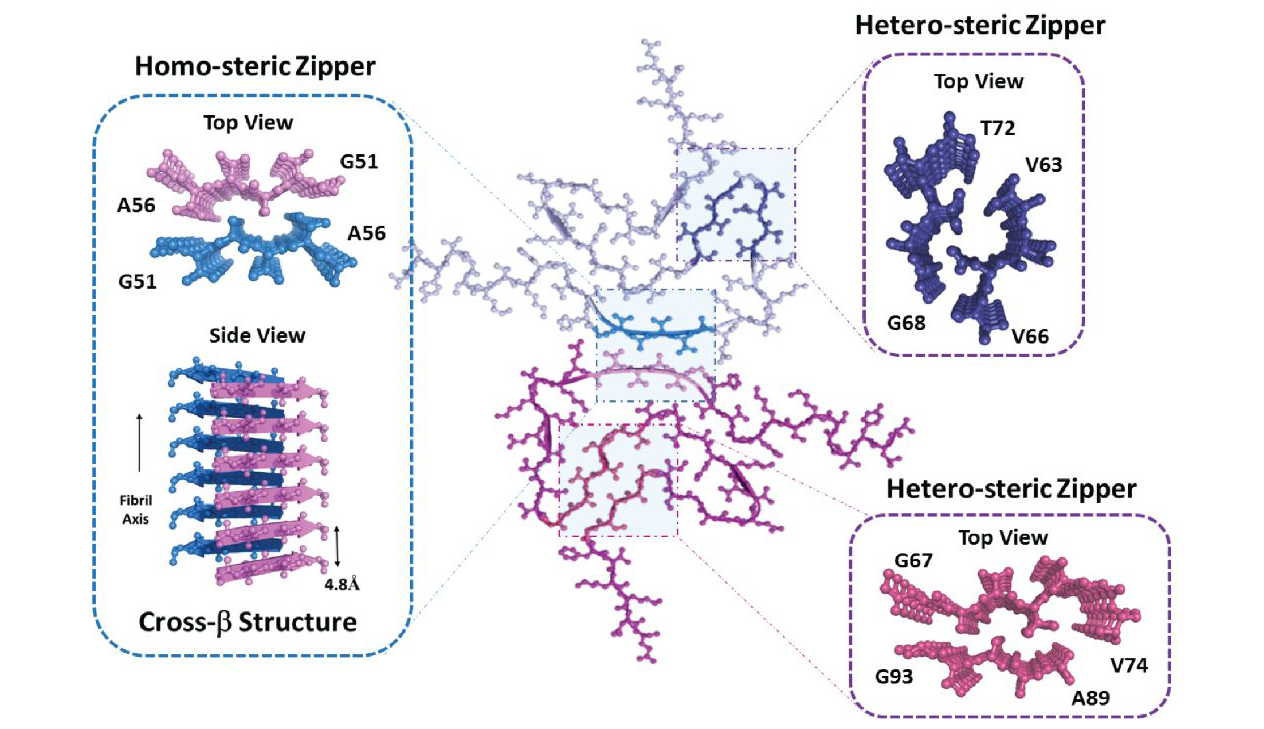
-

-
Second messenger Ap4A polymerizes target protein HINT1 to transduce signals in FcεRI-activated mast cells.Full Article
Yu J., Liu Z.Z., Liang Y.Y., Luo F., Zhang J., Tian C.P., Motzik A. Zheng M.M., Kang J.w., Zhong G.S., Liu C., Fang P.F., Guo M., Razin E.*, Wang J.*
Nat. Commun., 2019, 10, 4664(*corresponding author) 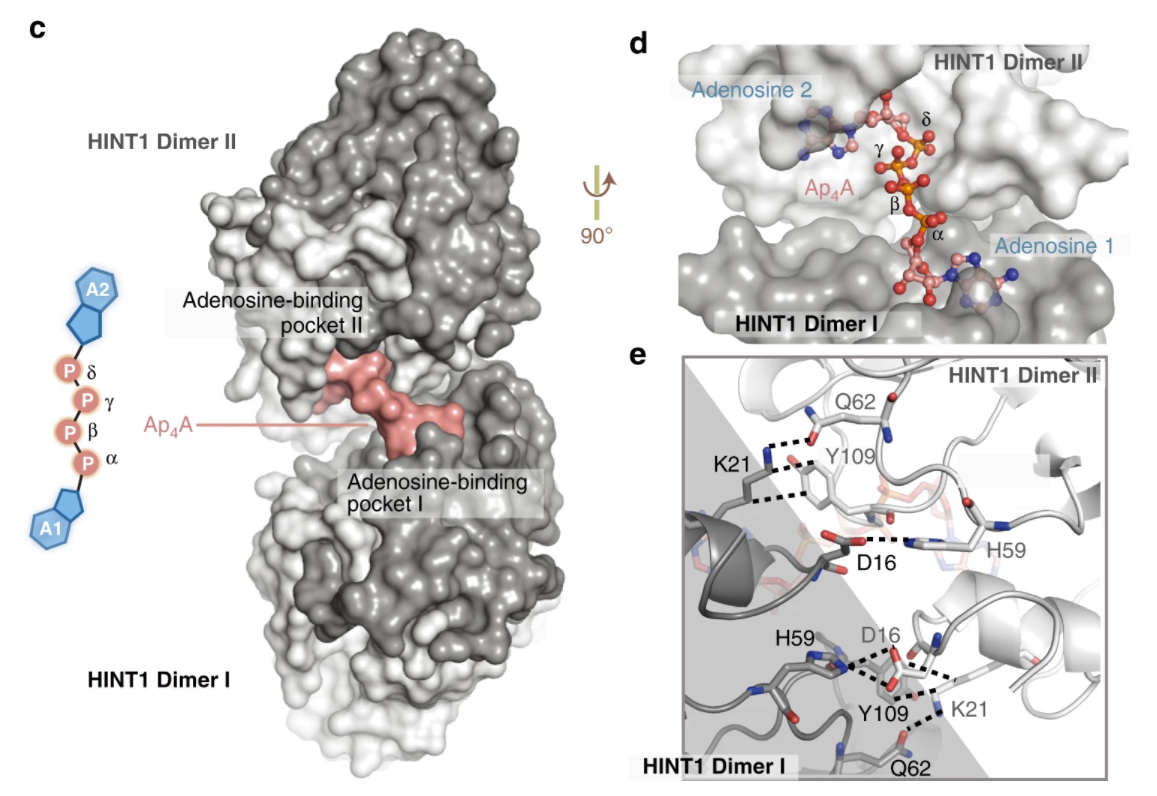
-

-
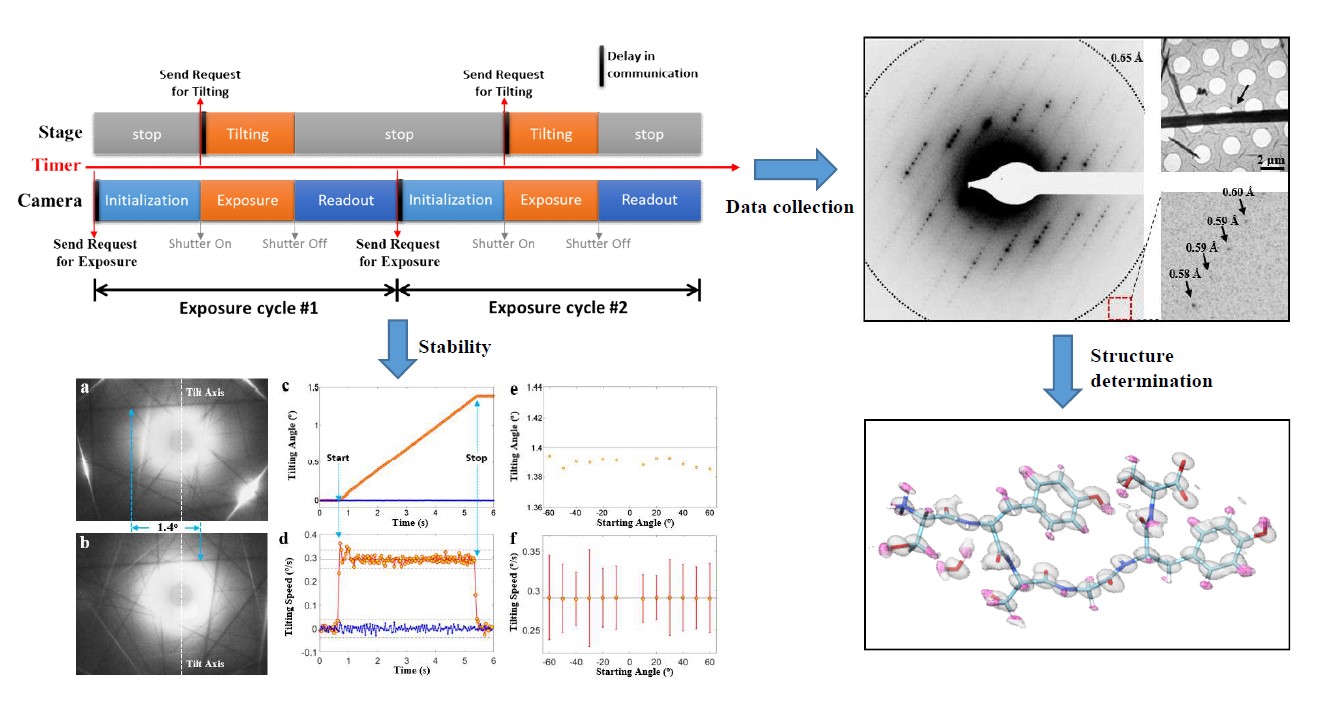
Full ArticleProgramming Conventional Electron Microscopes for Solving Ultrahigh-Resolution Structures of Small and Macro-Molecules.Zhou H., Luo F., Luo Z.P., Li D., Liu C.*, Li X.M.*Analytical Chemistry, 2019(*corresponding author)
-

-
Full ArticleExploiting mammalian low-complexity domains for liquid-liquid phase separation–driven underwater adhesive coatings.Cui M.K., Wang X.Y., An B.L., Zhang C., Gui X.R., Li K., Li Y.F., Ge P., Zhang J.H., Liu C., Zhang C.*Science Advances, 2019, 5(8), eaax3155(*corresponding author)

-

-
Structural basis for reversible amyloids of hnRNPA1 elucidates their role in stress granule assembly.Full Article
Gui X.R., Luo F., Li Y.C., Zhou H., Qin Z.H., Liu Z.Y., Gu J.G., Xie M.Y., Zhao K., Dai B., Shin W.S., He J.H., He L., Jiang L., Zhao M.L., Sun B., Li X.M., Liu C.*, Li D.*
Nat. Commun., 2019, 10(1), 2006(*corresponding author) 
-

-
Modular genetic design of multi-domain functional amyloids: insights into self-assembly and functional properties.Full Article
Cui M.K., Qi Q., Gurry T., Zhao T.X., An B., Pu J.H., Gui X.R., Cheng A.A., Zhang A.Y., Xun D.M., Becce M., Briatico-Vangosa F., Liu C., Lu T.K., Zhong C.
Chem Sci, 2019, 10(14), 4004–4014 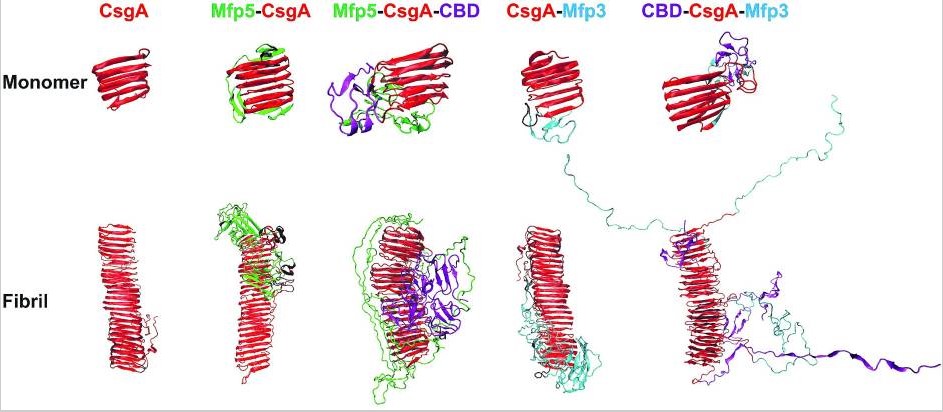
-

-
Different Heat Shock Proteins Bind α-Synuclein With Distinct Mechanisms and Synergistically Prevent Its Amyloid AggregationFull Article
Jia C.Y., Ma X.J., Liu Z.Y., Gu J.G., Zhang X., Li D.*, Zhang S.N.*
Front. Neurosci., 2019, 13, 1124(*corresponding author) 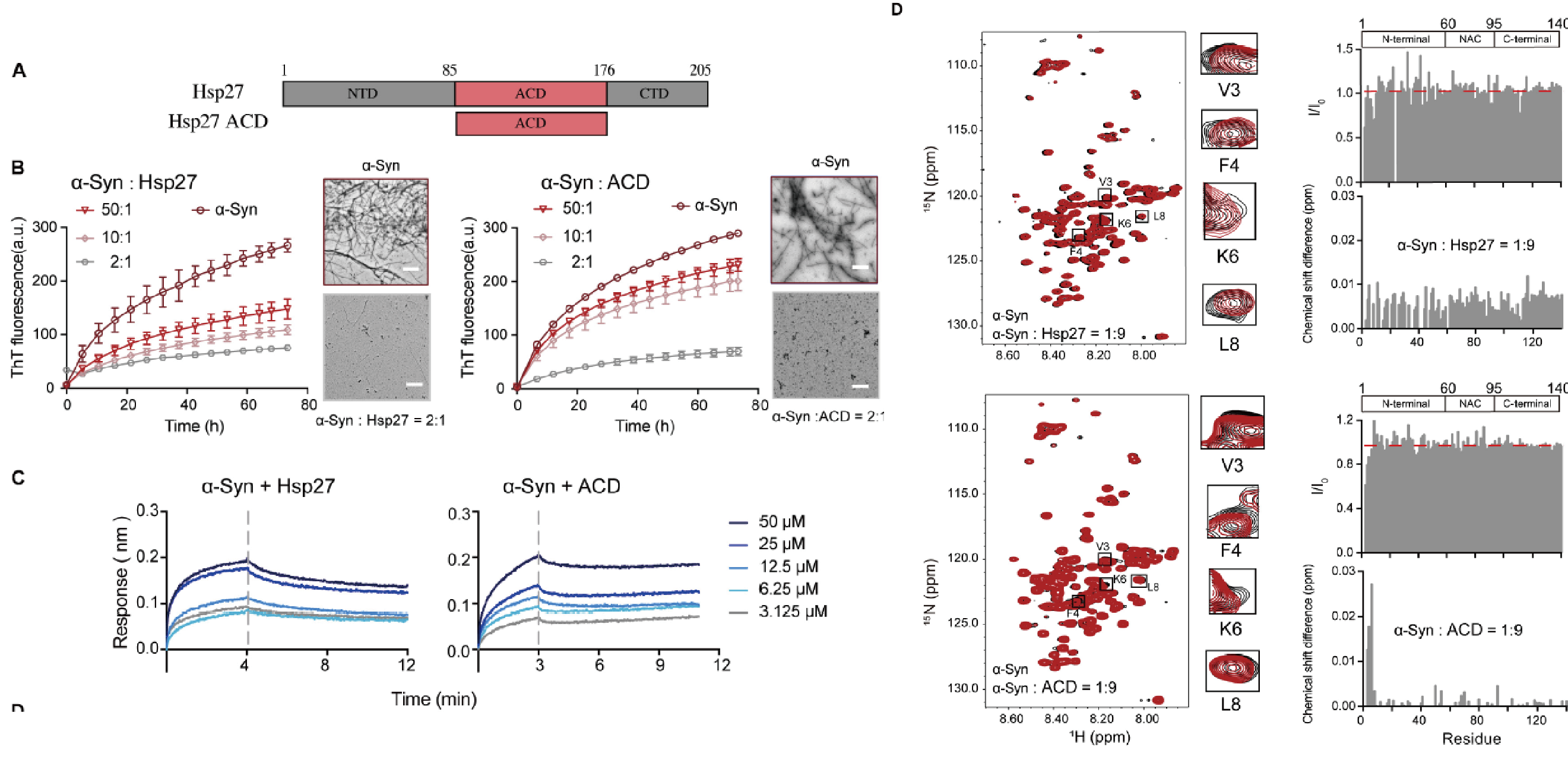
-

-
Structure-Based Peptide Inhibitor Design of Amyloid-β AggregationFull Article
Lu J.X., Cao Q.,Wang C.C., Zheng J.,Luo F.,Xie J.F., Li Y.C.,Ma X.J., He L., Eisenberg D., Nowick J., Jiang L.*, Li D.*
Front. Mol. Neurosci., 2019, 12, 54(*corresponding author) 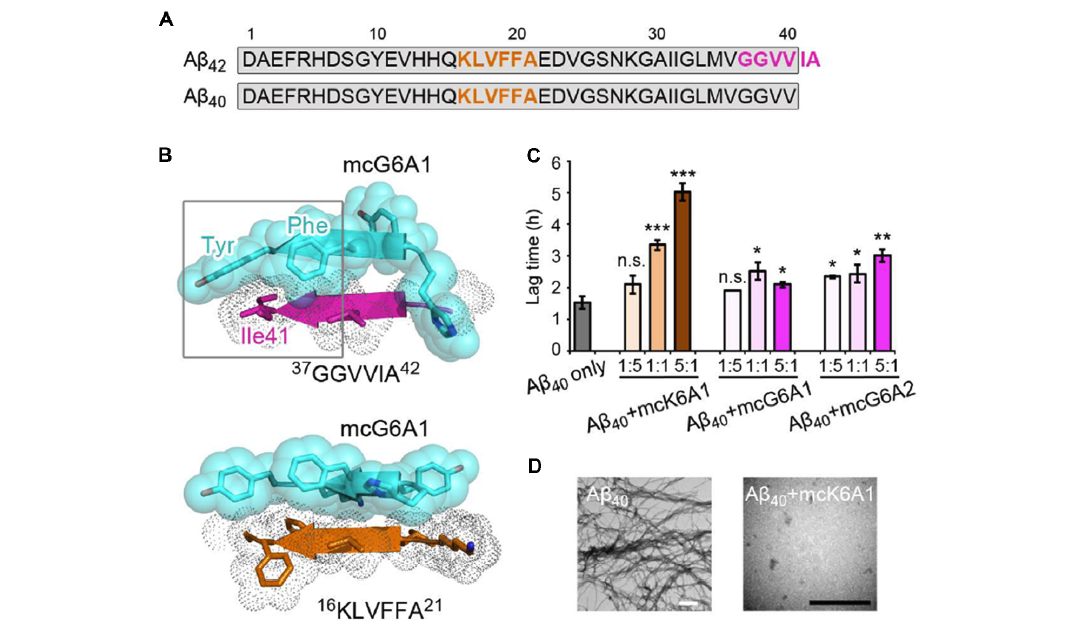
-

-
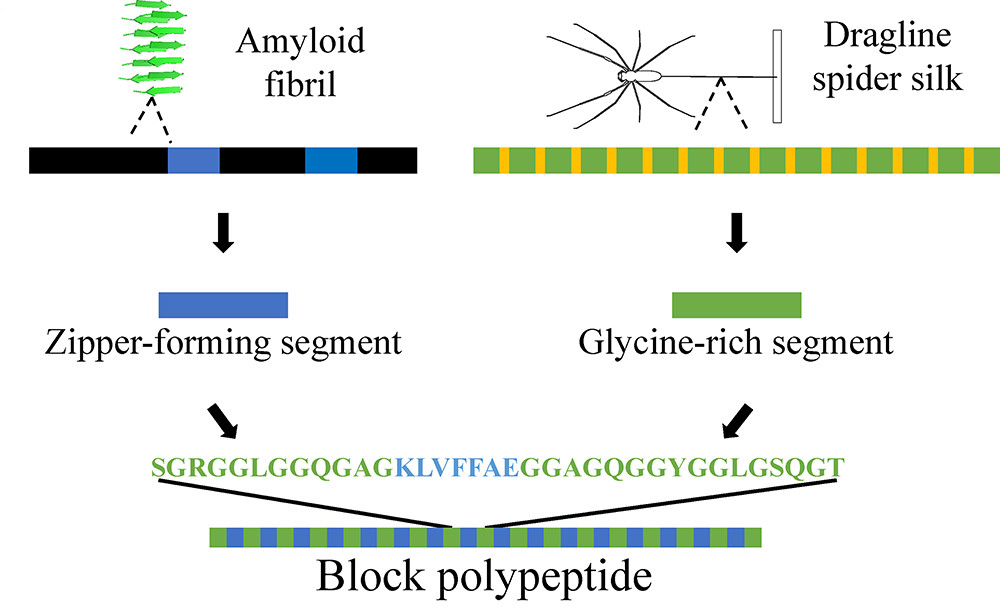
Full Article
Fibril Self-Assembly of Amyloid-Spider Silk Block Polypeptides.
Dai B., Sargent C.J., Gui X.R., Liu C., Zhang F.
Biomacromolecules, 2019
-

-
Full Article
New insights of poly(ADP-ribosylation) in neurodegenerative diseases: A focus on protein phase separation and pathologic aggregation. (Invited review)
Liu C.*, Fang Y.*
Biochem Pharmacol, 2019(*corresponding author) 
-

-
Full Article
Detecting single molecule dynamics on lipid membranes with quenchers-in-a-liposome FRET.
Ma D.F., Xu C.H., Hou W.Q., Zhao C.Y., Ma J.B., Huang X.Y., Jia Q., Ma L., Diao J.J., Liu C.*, Li M.*, Lu Y.*
Angew Chem Int Ed Engl, 2019,58(17), 5577-5581(* corresponding author) 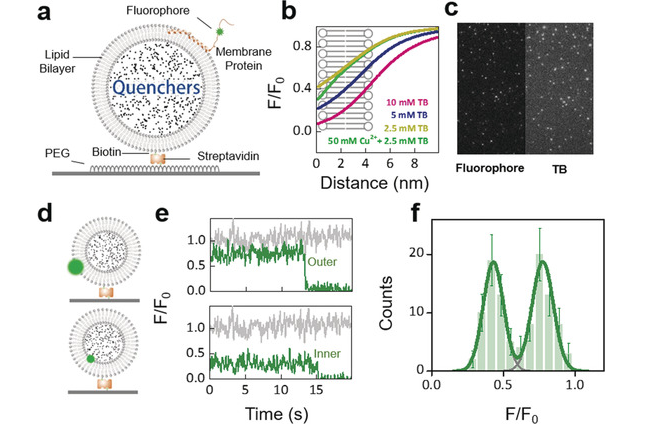
-
-
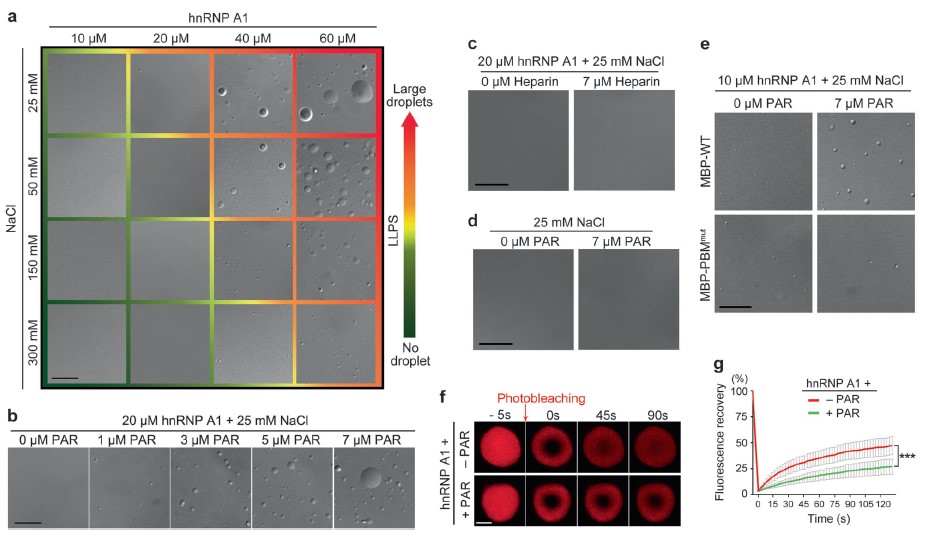
Full Article
PARylation regulates stress granule dynamics, phase separation, and neurotoxicity of disease-related RNA-binding proteins.
Duan Y.J.¶, Du A.Y.¶, Gu J.G.¶, Duan G., Wang C., Gui X.R., Ma Z.W., Qian B.T., Deng X., Zhang K., Sun L., Tian K.L., Zhang Y.Y., Jiang H., Liu C.*, Fang Y.S.*
Cell Research, 2019, 29, 233-247 (* corresponding author)
-
-
Full Article
Heat shock protein 104 (HSP104) chaperones soluble Tau via a mechanism distinct from its disaggregase activity.
Zhang X., Zhang S.N., Zhang L., Lu J.X., Zhao C.Y., Luo F., Li D., Li X.M.*, Liu C.*
J. Biol. Chem., 2019, 294(13), 4956-4965(* corresponding author)
-

-
Full Article
In-Cell NMR Study of Tau and MARK2 Phosphorylated Tau.
Zhang S.N.*¶, Wang C.C.¶, Lu J.X., Ma X.J., Liu Z.Y., Li D., Liu Z.J., Liu C.*
Int. J. Mol. Sci., 2018, 20(1), 90 (*corresponding author)
-

-
Full Article
Mechanistic insights into the switch of αB-crystallin chaperone activity and self-multimerization.
Liu Z.Y., Wang C.C., Li Y.C., Zhao C.Y., Li T.Z., Li D., Zhang S.N.*, Liu C.*
J. Biol. Chem., 2018, 293(38):14880-14890 (* corresponding author)
-

-
Full Article
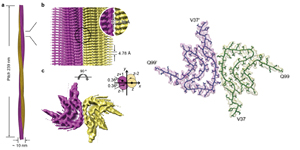
Amyloid fibril structure of α-synuclein determined by cryoelectron microscopy.
Li Y.W.¶, Zhao C.Y.¶, Luo F.¶, Liu Z.Y., Gui X.R., Luo Z.P., Zhang X., Li D.*, Liu C.*, Li X.M.*
Cell Research, 2018, 28, 897-903 (* corresponding author)
-

-
Full Article
Better Together: A Hybrid Amyloid Signals Necroptosis. (Invited preview)
Li D.*, Liu C.*
Cell, 2018, 173, 1068-1070. (* corresponding author)
-

-
Full Article
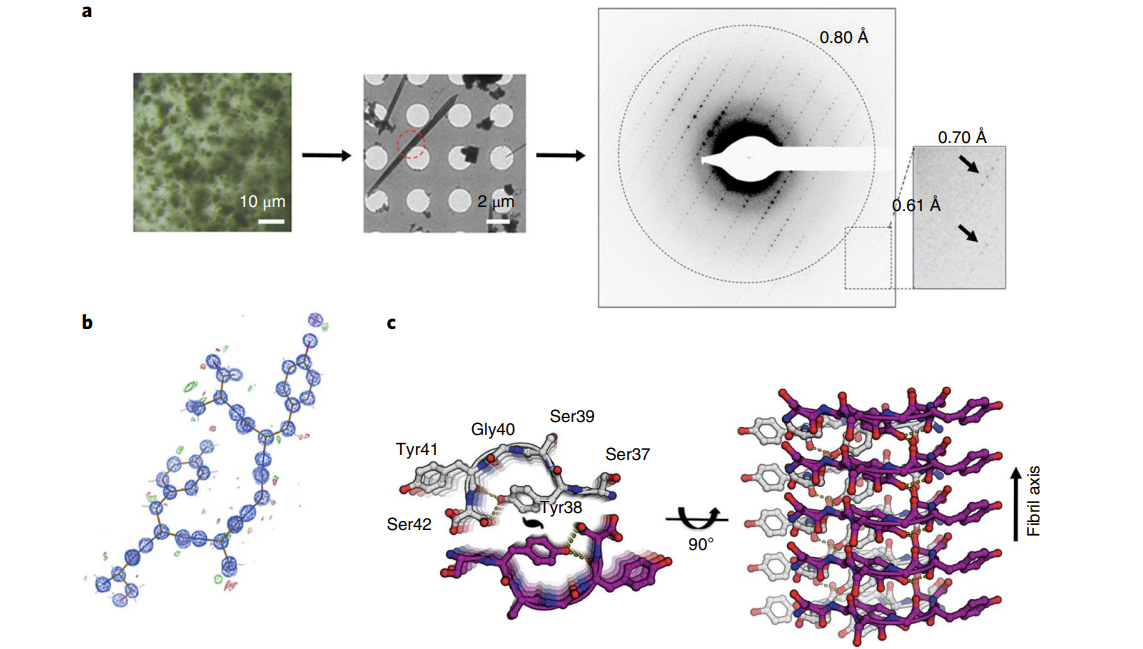
Atomic structures of two segments from FUS LC domain reveal reversible amyloid fibril formation.
Luo F.¶, Gui X.R.¶, Zhou H.¶, Gu J.G., Li Y.C., Liu X.Y., Zhao M.L., Li D.*, Li X.M.* and Liu C.*
Nat. Struct. Mol. Biol., 2018, 25, 341-346. (* corresponding author)
-

-
Full Article
Allosteric Inhibitors of SHP2 with Therapeutic Potential for Cancer Treatment.
Xie J.J., Si X.J., Gu S.L., Wang M.L., Shen J., Li H.Y., Shen J., Li D., Fang Y.J., Liu C.* and Zhu J.D.*
Journal of Medicinal Chemistry, 2017, 60, 10205-10219. (* corresponding author)

-
-
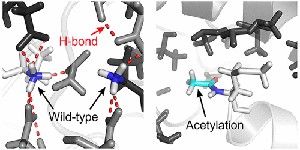
Full Article
N-Terminal Acetylation Preserves α-Synuclein from Oligomerization by Blocking Intermolecular Hydrogen Bonds.
Bu B., Tong X., Li D., Hu Y., He W., Zhao C., Hu R., Li X., Shao Y., Liu C., Zhao Q., Ji B., and Diao J.
ACS Chem Neurosci., 2017, 18, 2145-2151.
-

-
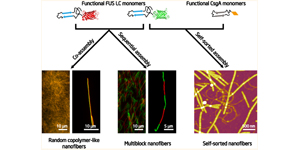
Full Article
Diverse Supramolecular Nanofiber Networks Assembled by Functional Low-Complexity Domains.
An B.L., Wang X.Y., Cui M.K., Gui X.R., Mao X.H., Liu Y., Li K., Chu C.F., Pu J.H., Ren S.S., Wang Y.Y., Zhong G.S., Lu T.K., Liu C. and Zhong C.
ACS Nano, 2017, 11:6985-6995.
-

-
Full Article
A structural view of αB-crystallin assembly and amyloid aggregation. (Invited review)
Liu Z.Y., Zhang S.N., Li D., and Liu C.*
Protein & Peptide Letters, 2017, 24, 1-7. (* corresponding author)

沪ICP备2022014231号-1


